Abstract
Background/Aims
Infectious complications are major concerns when treating patients with inflammatory bowel disease (IBD). This study evaluated clinical differences across countries/regions in the management of infectious diseases in patients with IBD.
Methods
A multinational online questionnaire survey was administered to participants at the 8th meeting of the Asian Organization for Crohn’s and Colitis. The questionnaire included questions regarding surveillance, diagnosis, management, and prevention of infection in patients with IBD.
Results
A total of 384 physicians responded to the questionnaire. The majority of Korean (n=70, 63.6%) and Chinese (n=51, 51.5%) physicians preferred vancomycin to metronidazole in the treatment of Clostridium difficile infection, whereas more than half of the Japanese physicians (n=62, 66.7%) preferred metronidazole. Physicians in Korea (n=88, 80.0%) and China (n=46, 46.5%) preferred a 3-month course of isoniazid and rifampin to treat latent tuberculosis infection, whereas most physicians in Japan (n=71, 76.3%) favored a 9-month course of isoniazid. Most Korean physicians (n=89, 80.9%) recommended hepatitis B virus (HBV) vaccination in patients lacking HBV surface antigen, whereas more than half of Japanese physicians (n=53, 57.0%) did not consider vaccination.
Conclusions
Differences in the diagnosis, prevention, and management of infections in patients with IBD across countries/regions reflect different prevalence rates of infectious diseases. This survey may broaden understanding of the real-world clinical settings across Asian countries/regions and provide information for establishing practical guidelines to manage patients with IBD.
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract that includes 2 specific diseases: ulcerative colitis (UC) and Crohn’s disease [1]. Although the incidence and prevalence of IBD were formerly lower in Asian than in Western countries, the number of Asian patients with IBD has increased markedly in recent decades [2,3]. Because an increasing percentage of patients with IBD are treated with immunomodulating agents, including corticosteroids, immunosuppressants, biologics, and small molecules, the risk of infectious complications has increased [4,5]. Moreover, the number of Asian patients treated with immunomodulating agents has increased [6,7]. Therefore, understanding the differences in the diagnosis, prevention, and treatment patterns of IBD-related infectious complications is important. Cytomegalovirus (CMV) colitis, Clostridium difficile infection (CDI), tuberculosis (TB), and chronic viral hepatitis are major infectious complications in patients with IBD.
International groups of IBD experts have established guidelines on the management of infectious diseases in patients with IBD, providing valuable advice to clinicians managing infectious complications in these patients [8-11]. These guidelines recommend assessing CMV colitis in patients with acute severe or steroid-refractory UC using several diagnostic methods, including tissue staining with hematoxylin and eosin, real-time polymerase chain reaction (PCR), and immunohistochemistry (IHC). Evaluation of CDI is also required in patients with acute severe UC, with the treatment options for CDI varying slightly among guidelines. Some guidelines recommend the use of vancomycin over metronidazole, whereas others recommend the use of both antibiotics to treat mild-to-moderate CDI. Guidelines also recommend that patients be screened for latent TB infection (LTBI) before treatment with anti-tumor necrosis factor-α (anti-TNF-α) agents to prevent the reactivation of LTBI. Patients positive for LTBI should be started on chemoprophylaxis for TB before starting anti-TNF-α treatment. The prevalence of hepatitis B virus (HBV) infection in patients with UC is similar to that in the general population [12]. Guidelines have suggested that HBV serology should be performed before initiating treatment with immunomodulators or biologics. Patients with IBD who test positive for HBV surface antigen (HBsAg) should receive antiviral agents, whereas seronegative patients should be vaccinated for HBV [13]. Screening for TB and HBV is especially important in Asian countries due to their high risks of TB and chronic HBV hepatitis [14,15]. Indeed, guidelines recommend that Asian patients with IBD who require not only immunosuppressants and/or biologics but also corticosteroids undergo screening tests for TB and HBV.
To provide an in-depth understanding of IBD management in real-world clinical settings in Asian countries, the board of the Asian Organization of Crohn’s and Colitis (AOCC) administered an online questionnaire to physicians [16-18]. The survey was designed to evaluate differences in the diagnosis and management of infectious diseases in patients with IBD across these countries, thereby establishing optimal management guidelines.
An online questionnaire survey was developed through the collaboration of the AOCC, the International Academic Exchange Committee, Scientific Committee, and IBD Research Group of the Korean Association for the Study of Intestinal Diseases. Members of these organizations selected and reviewed each question to ensure that it reflected real-world clinical settings of Asian patients with IBD. Questionnaires were sent via SurveyMonkey to approximately 16,000 potential respondents, consisting of multinational members of the AOCC with available email addresses. The online survey, which consisted of 95 items, was available from September 16 to November 13, 2020. Respondents were asked to enter their baseline characteristics, including country/region, sex, type of practice, specialty, and medical experience (a total of 8 items). They were also asked to complete questions assessing real-world clinical settings related to IBD, including questions related to the diagnosis (17 items), treatment (33 items), infection (22 items), and vaccination (15 items) of patients with IBD; questions regarding infections are described in detail in the Supplementary Material. Some questions permitted multiple responses, whereas others permitted single responses. Categorical variables were analyzed using cross-tabulations and the chi-square or Fisher exact tests using SPSS version 25.0 statistical software (IBM Corp., Armonk, NY, USA). P-values < 0.05 were considered statistically significant for the questions that permitted single responses.
The survey included 384 physicians from multiple countries/regions, 253 men (65.9%) and 89 women (34.1%), and the survey response rate was 2.4% and margin of error was 4.9% at the 95% confidence level. Most respondents were from Asian countries/regions, but several were from other countries/regions, such as Australia, Egypt, and Lebanon. Among the Asian countries/regions, the highest numbers of responders were from Korea (n=110, 28.6%), followed by China (n=99, 25.8%) and Japan (n=93, 24.2%). Smaller numbers of responders were from Vietnam (n=8, 2.1%), Indonesia (n=7, 1.8%), India (n=6, 1.5%), the Philippines (n=4, 1.0%), whereas 57 (14.8%) from other Asian countries. Therefore, this analysis focused on the responses of physicians from Korea, China, and Japan. Most respondents (n=335, 87.2%) worked at academic teaching hospitals, and more than half were gastroenterologists specializing in IBD (n=211, 55.0%).
Most respondents considered CMV IHC when punch-out ulcerations were discovered on endoscopy (n=224, 58.3%), as well as when glucocorticoid resistance was suspected in patients with IBD (n=187, 48.7%) (Fig. 1A). Most physicians who suspected CMV colitis in patients with IBD confirmed the diagnosis by IHC of endoscopic biopsy samples (n=310, 80.7%), followed by hematoxylin and eosin staining or measurement of CMV DNA level in endoscopic biopsy samples (Fig. 1B). Tissue-based tests were preferred over blood-based assays, such as blood CMV immunoglobulin M, immunoglobulin G, antigenemia assay, and CMV DNA tests. Most responders treated CMV colitis by intravenous injection of ganciclovir (n=331, 86.2%) (Fig. 1C). Although some physicians from China and other countries/regions treated CMV colitis with foscarnet sodium, none of the physicians from Korea or Japan chose this agent. More than half of the respondents did not consult infection specialists before prescribing antiviral agents (n=232, 60.4%).
Most physicians recommended CDI tests in patients with active IBD (n=278, 72.4%), as well as in patients unresponsive to corticosteroid treatment (n=194, 50.52%) (Fig. 2A). In diagnosing CDI, the stool C. difficile toxin A/B assay was the most frequently performed test, regardless of the country/region (n=349, 90.9%), followed by stool C. difficile culture (n=184, 48.0%) (Fig. 2B). Approximately 52.8% of the respondents preferred vancomycin to metronidazole in the treatment of CDI (n=199) (Fig. 2C). More than two-thirds of the Korean physicians prescribed vancomycin (n=70, 63.6%), whereas more than two-thirds of the Japanese physicians preferred metronidazole (n=62, 66.7%). One hundred and fifty-nine respondents (41.0%) considered omitting immunomodulators in the treatment with CDI. More than half the respondents did not consider fecal microbiota transplantation (FMT) for refractory CDI (n=233, 60.7%), with these percentages differing among countries/regions.
More than 98% of the respondents considered TB screening in patients with IBD at the time of diagnosis and when considering the use of biologics, small molecules, or immunomodulators, whereas only 4 (1.0%) did not include TB screening (Fig. 3A). Of the respondents who screened their patients for TB, 335 (92.5%) preferred interferon-γ release assays, followed by chest radiography (n=333, 86.7%) (Fig. 3B). The choice of anti-TB regimen for LTBI also differed among the countries/regions (Fig. 3C). Physicians from Korea (n=88, 80.0%) and China (n=46, 46.5%) generally treated LTBI with a 3-month combination of isoniazid and rifampin, whereas those from Japan generally preferred isoniazid alone for 9 months (n=71, 76.3%). A few physicians chose 4 months of rifampin or 12 doses of isoniazid and rifapentine. Upon diagnosis of active TB in patients with IBD, 81.0% of the respondents discontinued biologics or small molecules, regardless of the country/region. Of the respondents, 34.6% (n=133) restarted biologics after the end of anti-TB therapy, whereas 26.8% (n=103) restarted biologics 3 months after the initiation of anti-TB therapy.
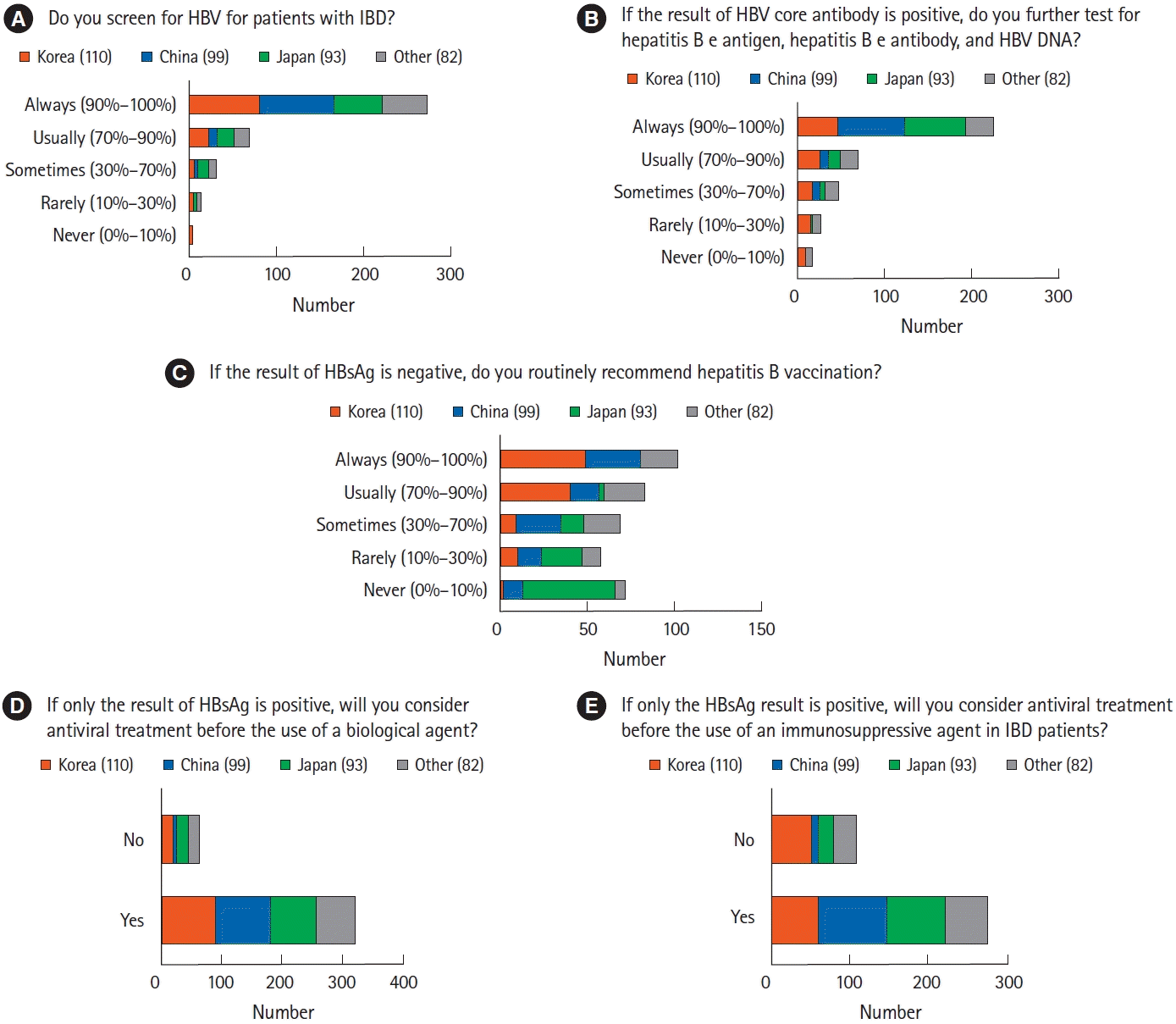
More than 70% of the respondents always screened for HBV in patients with IBD (n=273, 71.1%), whereas only 15 respondents (3.9%) never or rarely screened these patients (Fig. 4A). Most respondents utilized HBsAg tests as routine HBV screening before IBD treatment (n=373, 97.1%), followed by HBV core antibody (n=304, 79.2%) and HBV surface antibody (n=292, 76.0%) tests. More than half of the respondents always underwent additional HBV tests, such as hepatitis B e antigen, hepatitis B e antibody, and HBV DNA tests (Fig 4B). Respondents from different countries/regions differed significantly in choosing to vaccinate HBsAg-negative patients (P<0.001) (Fig 4C). Specifically, physicians from Korea (n=89, 80.9%) and China (n=48, 48.5%) considered HBV vaccination in HBsAg-negative patients with IBD. By contrast, physicians from Japan (n=53, 57.0%) did not highly recommend HBV vaccination to HBsAg-negative patients. Most respondents agreed that antiviral treatment should be administered to IBD patients positive for HBsAg before initiating treatment with biologics (n=321, 83.6%) or immunosuppressive agents (n=276, 71.9%) (Fig. 4D and E). In addition, about two-thirds of respondents considered hepatitis C virus treatment for hepatitis C virus antibody-positive patients in the active stage of IBD (n=260, 67.7%).
The present study surveyed real-world clinical practices adopted by Asian physicians when managing infections in patients with IBD. Because the prevalence rate of each infectious disease differs in Asian and Western countries, the rates of infectious complications also differ in these countries. Screening for LTBI and HBV infection is important in Asian countries where these infections are endemic [19-21]. By contrast, Western physicians screen IBD patients for human immunodeficiency virus (HIV) infection, as its prevalence is high and HIV transmission is a major public health issue in Western countries [22]. The present survey found that, despite the general similarities across Asian countries/regions in the diagnosis, prevention, and treatment of infectious diseases, several factors differed.
Asian physicians suspected CMV colitis in patients with punch-out ulcerations on endoscopic examination or glucocorticoid resistance. The diagnosis of CMV colitis in most Asian patients is based on the confirmation of CMV infection by IHC of endoscopically biopsied specimens. Although ganciclovir is the most widely used antiviral agent for CMV colitis, regardless of country/region, this agent requires intravenous administration, making it unsuitable for outpatient treatment. Valganciclovir, an orally administrated ganciclovir prodrug, is regarded as an alternative to ganciclovir for CMV colitis [23]. Oral valganciclovir is also less costly than ganciclovir. Chinese guidelines recommend foscarnet as well as ganciclovir as a treatment for CMV colitis [24], with more physicians from China prescribing foscarnet than physicians from other countries.
Although the symptoms of CDI and IBD flares, such as fever, abdominal pain, diarrhea, and hematochezia, are similar, their treatments differ markedly [25]. Because the risk of CDI is greater in patients with than without IBD [26], combined CDI should be considered in patients with IBD. Although Asian guidelines recommend tests for CDI for IBD patients who are unresponsive to glucocorticoid therapy [24,27], all active-stage IBD patients in real-world clinical settings are suspected of having combined CDI. Nucleotide PCR assays are more accurate and faster than other tests for CDI but are too sensitive, with a risk of overdiagnosis [28,29]. Asian guidelines recommend that CDI be diagnosed based on the result of stool C. difficile toxin A/B assays [24,27], with most Asian physicians in the current survey using these assays to diagnose CDI. Although survey participants did not prefer the glutamate dehydrogenase antigen assay for detecting CDI in patients with IBD, the glutamate dehydrogenase antigen assay is recommended for initial screening [30].
A prospective randomized trial showed that the efficacies of vancomycin and metronidazole were similar in patients with mild CDI, whereas vancomycin was superior to metronidazole in patients with severe CDI [31]. Metronidazole, however, can also be prescribed for the treatment of mild CDI in patients with IBD. In the present survey, Korean and Chinese physicians preferred vancomycin, whereas Japanese physicians preferred metronidazole. By contrast, another study of CDI treatment in Japan found that vancomycin was prescribed more frequently than metronidazole [32]. Although FMT is safe and effective for recurrent or refractory CDI in patients with IBD [33,34], the present questionnaire did not include items regarding FMT.
The prevalence of LTBI is approximately twice as high in Asian as in Western countries [35]. The prevalence of LTBI in Korea decreased to less than 50% in the 1990s as the number of active TB cases declined., whereas the prevalence of TB in China remains high despite national efforts to control it [36,37]. Based on regional differences in LTBI risk, the current survey revealed that Asian physicians are involved in early screening, prevention, and prophylaxis of TB. Distinguishing intestinal TB from CD is challenging, with delays in anti-TB treatment having serious consequences in some patients [38]. In addition, the risks of active TB and reactivation of LTBI were higher in patients with IBD who were treated with immunomodulators and biologics, especially anti-TNF-α therapy [39,40]. All potential patients treated with immunomodulators and biologics should be screened for LTBI [41], and most Asian physicians who participated in this survey reported performing TB screening before the initiation of biologics or small molecules. Korean guidelines recommend a 6- or 9-month course of isoniazid, a 3-month course of isoniazid and rifampin, or a 3-month course of isoniazid and rifapentine for LTBI [42], whereas Chinese guidelines recommend only 3-month regimens [24]. Adherence to 3-month regimens is high owing to the relatively short treatment period, whereas the single-drug regimens can reduce potential drug-drug interactions and complications. Participants from Korea and China in the present survey preferred the 3-month course of isoniazid and rifampin for latent TB, whereas participants from Japan preferred the 9-month course of isoniazid. The results of this survey reflect the prevalence rates of TB in each country. High treatment adherence is important in countries with a high TB risk, whereas drug complications are important in countries with a low TB risk.
HBV is highly endemic in most Asian countries, with a prevalence of over 8%, but is less prevalent in Western countries, with rates below 2% [43]. Asian physicians, regardless of country/region, agreed with the importance of screening for HBV in patients with IBD and prescribed antiviral agents to patients positive for HBsAg. However, the importance of vaccinating, HBsAg-negative patients differed among respondents across Asian countries/regions. Most physicians from Korea and China regarded vaccinating HBsAg-negative patients as important, whereas those from Japan did not, in agreement with results showing that the prevalence rates of HBV infection and chronic HBV hepatitis were higher in Korea and China than in Japan [44]. Korean guidelines recommend HBV vaccination for HBV-seronegative patients with IBD, regardless of the severity of immunodeficiency. By contrast, HBV vaccination is uncommon in Japan. Japan implemented selective HBV vaccination only for infants born to mothers who were HBV carriers and started routine HBV vaccination of infants in 2016 [45,46]. Japanese guidelines recommend HBV vaccination in patients with IBD receiving immunosuppressive therapy because substantial numbers of unvaccinated individuals are at risk of sexually transmitted HBV [47].
This study had several advantages, including its analysis of real-world clinical settings related to infectious diseases in Asian patients with IBD. This online questionnaire survey recruited a large number of Asian physicians, more than half of whom were gastroenterologists specializing in IBD, suggesting that the survey findings reflect real-world clinical settings in these Asian countries/regions. Moreover, in contrast to a previous study based on questionnaires administered to IBD investigators [48], the present study included a large number of Asian physicians from various countries. This present finding may also offer insights into the management of infectious complications in Asian patients with IBD that are distinct from those observed in Western patients with IBD [49,50]. Asian IBD specialists have focused on screening, diagnosis, and treatment of TB and HBV infection because these diseases are highly endemic in Asian but not in Western countries.
In conclusion, the present study revealed that the prevention, diagnosis, and treatment of infectious complications in patients with IBD are similar in Asian countries. However, some differences were observed that might reflect the differences in real-world clinical settings in each country. The results of this questionnaire survey conducted by the AOCC may enhance understanding regarding the management of IBD in Asian countries/regions and may aid in the development of realistic guidelines for managing infectious complications in Asian patients with IBD.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary Material
Supplementary materials are available at the Intestinal Research website (https://www.irjournal.org).
Supplementary Material
Detailed explanations of the questions about infections in patients with inflammatory bowel disease
REFERENCES
1. Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008; 14:354–377.

2. Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. 2022; 20:159–164.

3. Ye BD, Hong SN, Seo SI, et al. Changes in the long-term prognosis of Crohn’s disease between 1986 and 2015: the population-based Songpa-Kangdong inflammatory bowel disease cohort study. Gut Liver. 2022; 16:216–227.

4. Annese V, Duricova D, Gower-Rousseau C, Jess T, Langholz E. Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the Epidemiology Committee of ECCO. J Crohns Colitis. 2016; 10:216–225.

5. Barberio B, Savarino EV, Card T, et al. Incidence comparison of adverse events in patients with inflammatory bowel disease receiving different biologic agents: retrospective long-term evaluation. Intest Res. 2022; 20:114–123.

6. Singh A, Mahajan R, Kedia S, et al. Use of thiopurines in inflammatory bowel disease: an update. Intest Res. 2022; 20:11–30.

7. Hibi T, Kamae I, Pinton P, et al. Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis. Intest Res. 2021; 19:53–61.

8. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.

9. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018; 113:481–517.

10. Kucharzik T, Ellul P, Greuter T, et al. ECCO Guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021; 15:879–913.

11. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.

12. Giri S, Agrawal D, Afzalpurkar S, et al. Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res. 2023; 21:392–405.

13. Lee JM, Wei SC, Lee KM, et al. Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver. 2022; 16:396–403.

14. Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. J Gastroenterol Hepatol. 2019; 34:1296–1315.

15. Kang EA, Cheon JH. Antiviral prophylaxis against hepatitis B virus in patients treated with anti-tumor necrosis factor α agents for inflammatory bowel disease. Gut Liver. 2022; 16:501–502.

16. Song HK, Lee KM, Jung SA, et al. Quality of care in inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:240–247.

17. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:224–230.

18. Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:231–239.

19. Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020: reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021; 113(Suppl 1):S7–S12.
20. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018; 67:1–31.

21. Lernout T, Hendrickx G, Vorsters A, Mosina L, Emiroglu N, Van Damme P. A cohesive European policy for hepatitis B vaccination, are we there yet? Clin Microbiol Infect. 2014; 20 Suppl 5:19–24.

22. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468.

23. Cvetković RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005; 65:859–878.
24. Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association. Evidence-based consensus on opportunistic infections in inflammatory bowel disease (republication). Intest Res. 2018; 16:178–193.
25. Tang YM, Stone CD. Clostridium difficile infection in inflammatory bowel disease: challenges in diagnosis and treatment. Clin J Gastroenterol. 2017; 10:112–123.

26. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008; 103:1443–1450.

27. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017; 15:7–37.

28. Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009; 47:3211–3217.

29. Bélanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG. Rapid detection of Clostridium difficile in feces by real-time PCR. J Clin Microbiol. 2003; 41:730–734.
30. Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021; 116:1124–1147.

31. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007; 45:302–307.

32. Yamagishi Y, Mikamo H. Recent epidemiology of Clostridium difficile infection in Japan. Jpn J Antibiot. 2015; 68:345–358.
33. Allegretti JR, Kelly CR, Grinspan A, Mullish BH, Kassam Z, Fischer M. Outcomes of fecal microbiota transplantation in patients with inflammatory bowel diseases and recurrent Clostridioides difficile infection. Gastroenterology. 2020; 159:1982–1984.

34. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis. 2020; 26:1415–1420.

35. Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019; 54:1900655.

36. Zhu S, Xia L, Yu S, Chen S, Zhang J. The burden and challenges of tuberculosis in China: findings from the Global Burden of Disease Study 2015. Sci Rep. 2017; 7:14601.

37. World Health Organization (WHO). Global tuberculosis report 2020 [Internet]. c2020 [cited 2023 May 18]. https://www.who.int/publications/i/item/9789240013131.
38. Limsrivilai J, Pausawasdi N. Intestinal tuberculosis or Crohn’s disease: a review of the diagnostic models designed to differentiate between these two gastrointestinal diseases. Intest Res. 2021; 19:21–32.

39. Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008; 27:19–30.

40. Vaughn BP, Doherty GA, Gautam S, Moss AC, Cheifetz AS. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012; 18:1057–1063.

41. Riestra S, Taxonera C, Zabana Y, et al. Performance of screening strategies for latent tuberculosis infection in patients with inflammatory bowel disease: results from the ENEIDA registry of GETECCU. J Clin Med. 2022; 11:3915.

42. Lee SH. Diagnosis and treatment of latent tuberculosis infection: the updated 2017 Korean Guidelines. Korean J Med. 2018; 93:509–517.

43. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019; 16:57–73.

44. Merican I, Guan R, Amarapuka D, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000; 15:1356–1361.

45. Sagami S, Kobayashi T, Hibi T. Prevention of infectious diseases due to immunosuppression and vaccinations in Asian patients with inflammatory bowel disease. Inflamm Intest Dis. 2018; 3:1–10.

46. Ujiie M, Sasaki K, Yoshikawa N, Enami T, Shobayashi T. Introduction of a hepatitis B vaccine into the national routine immunisation programme of Japan. Lancet Infect Dis. 2016; 16:1325.

47. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.

48. Yang H, Ran Z, Jin M, Qian JM. Current status of opportunistic infection in inflammatory bowel disease patients in Asia: a questionnaire-based multicenter study. Gut Liver. 2022; 16:726–735.

49. Pan Z, Zhang J, Bu Q, et al. The gap between global tuberculosis incidence and the first milestone of the WHO end tuberculosis strategy: an analysis based on the global burden of disease 2017 database. Infect Drug Resist. 2020; 13:1281–1286.
Fig. 1.
Management of cytomegalovirus (CMV) infection in patients with inflammatory bowel disease (IBD). (A) The clinical circumstances when physicians suspected CMV colitis. (B) The methods for diagnosis of CMV colitis. (C) Differences among physicians from various countries in their management of CMV colitis. Subtle differences were observed among countries/regions in the preferred antiviral agents used to treat CMV infection. H&E, hematoxylin and eosin; Ig, immunoglobulin; IV, intravenous; PO, by mouth.
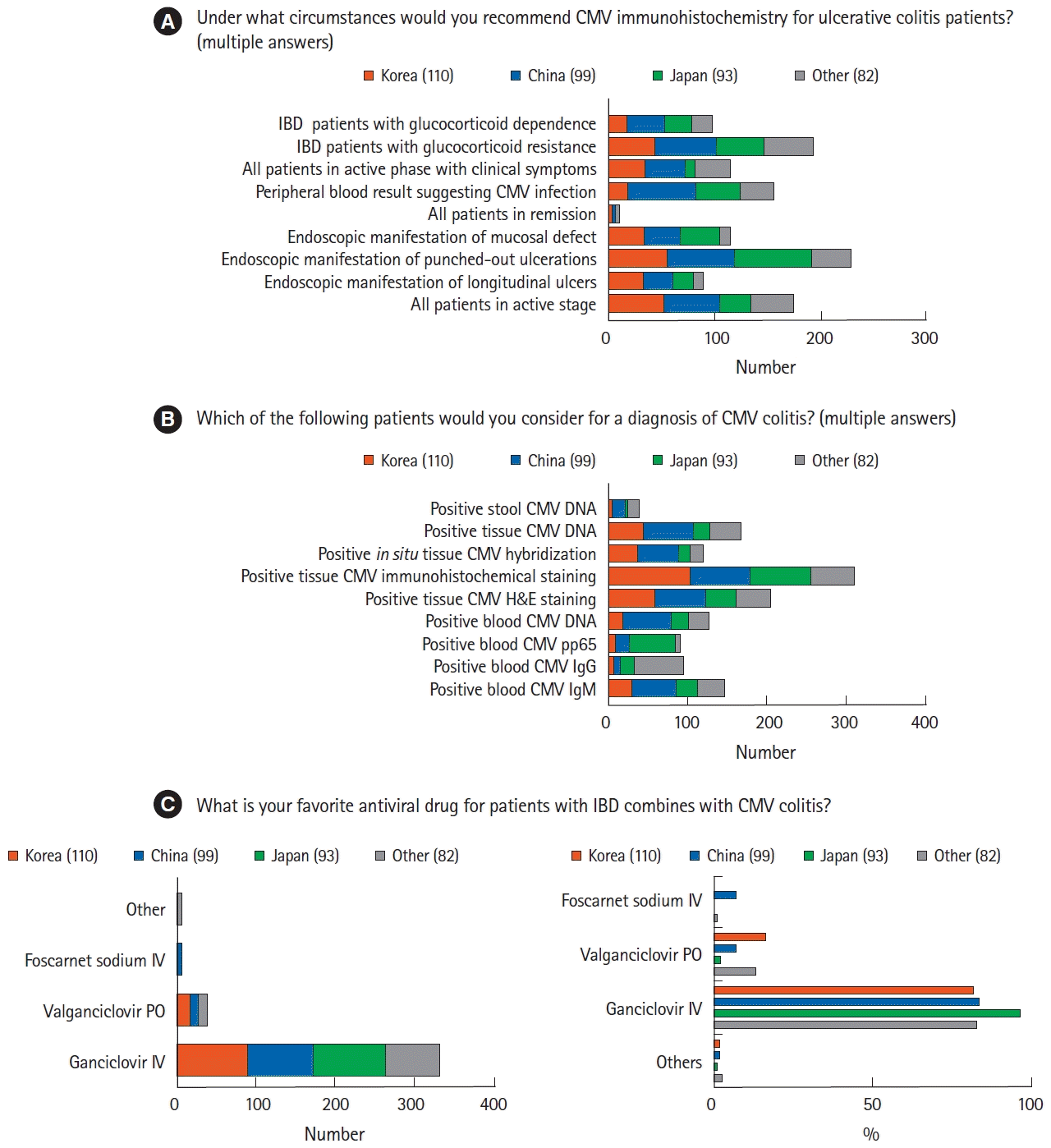
Fig. 2.
Diagnosis and treatment of Clostridium difficile infection (CDI) in patients with inflammatory bowel disease (IBD). (A) Rates of evaluation by Asian physicians of CDI in patients with IBD. Most physicians reported assessing CDI in all patients with active stage IBD. (B) Rates of diagnostic assays for CDI showing that the stool C. difficile toxin A/B test was the most widely used. (C) Rates of agents used in various Asian countries to treat CDI in IBD patients. Physicians from Korea and China preferred vancomycin is preferred over metronidazole, whereas physicians from Japan preferred metronidazole. PCR, polymerase chain reaction; GDH, glutamate dehydrogenase.
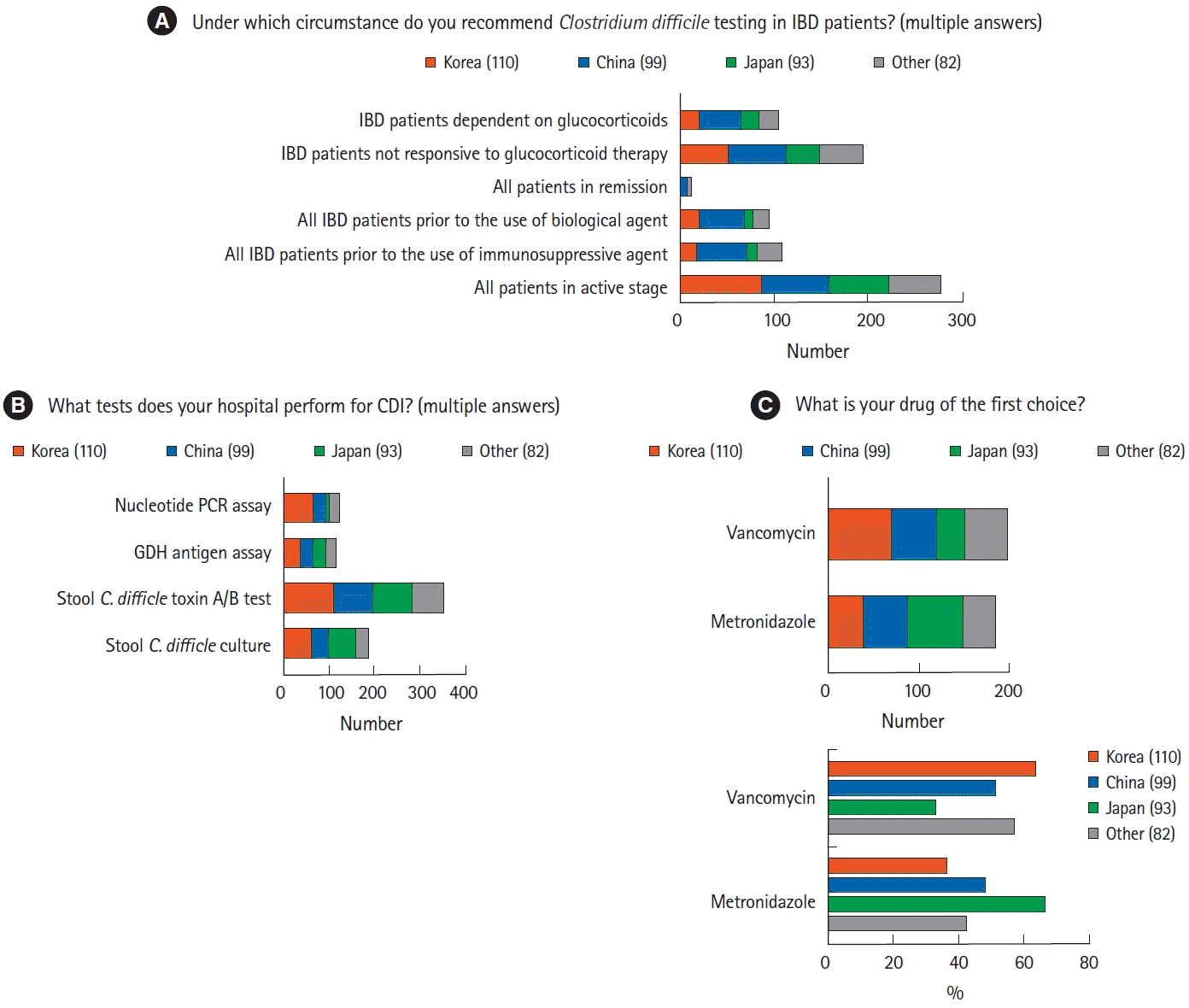
Fig. 3.
Diagnosis and treatment of tuberculosis (TB) in patients with inflammatory bowel disease (IBD). (A) Rates of evaluation by Asian physicians of active or latent TB in patients with IBD. Most physicians reported assessing TB before initiating treatment with biologics or small molecules. (B) Methods preferred by Asian physicians for the assessment of latent TB infection (LTBI). Interferon-γ release assay was the most preferred method, followed by chest radiography. (C) Regimens preferred by Asian physicians for the treatment of LTBI showed that the combination of isoniazid and rifampin for 3 months was the most frequently used regimen. IGRA, interferon-γ release assay; PPD, purified protein derivative.
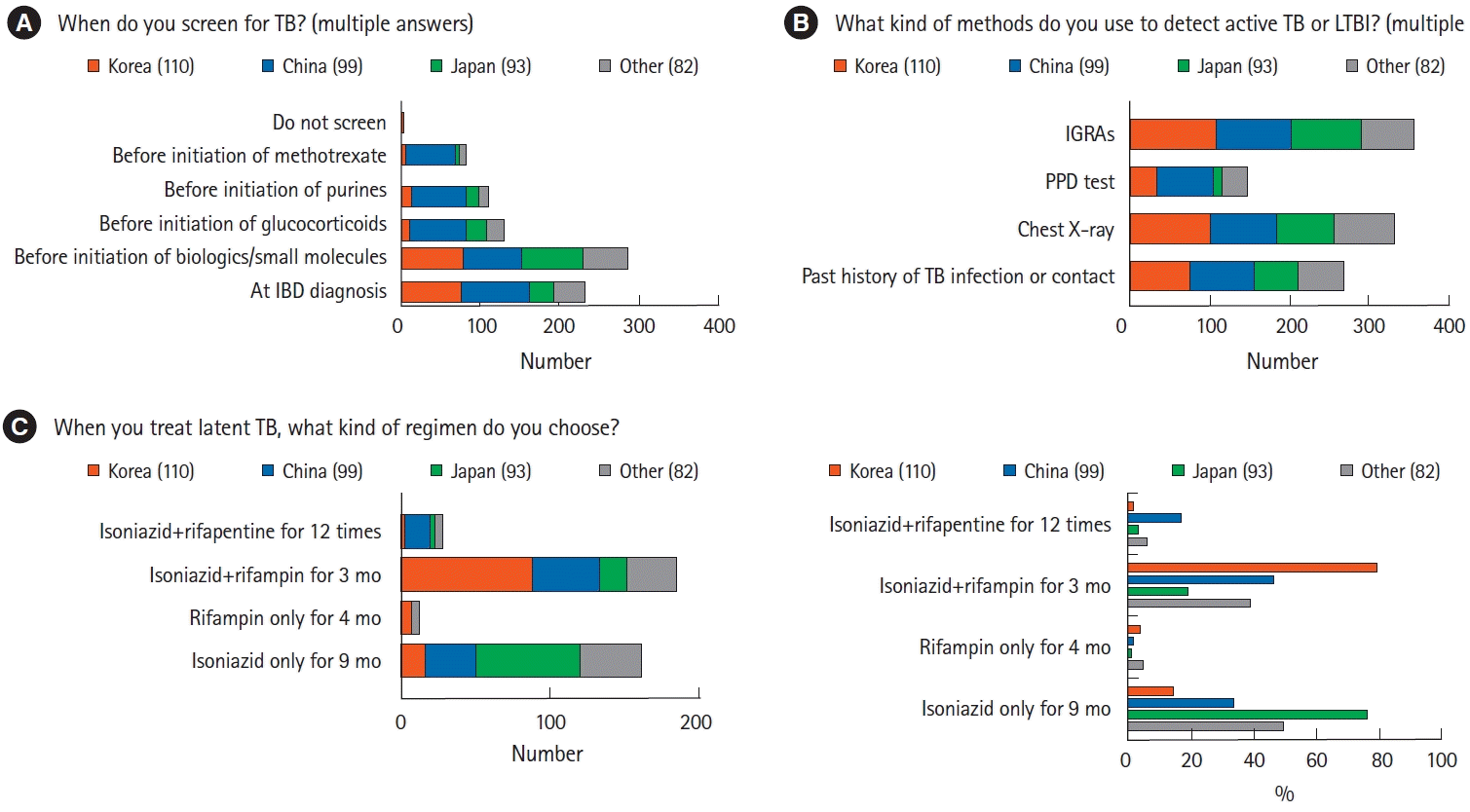
Fig. 4.
Management of chronic hepatitis B (CHB) and prevention of hepatitis B virus (HBV) reactivation in patients with inflammatory bowel disease (IBD). (A) Rates of HBV screening by Asian physicians of patients with IBD. (B) CHB diagnostic tests in patients positive for HBV core antibody. (C) Differences among physicians from various countries in their management of HBV surface antigen (HBsAg)-negative patients. Unlike Japanese physicians, Korean and Chinese physicians tended to vaccinate IBD patients for HBV. (D) Rates of antiviral treatment before initiation of biologics in patients with positive HBsAg. (E) Rates of antiviral treatment before initiation of immunosuppressants in patients with positive HBsAg.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download