1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.

2. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.

3. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.
4. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology. 2013; 145:158–165.
5. Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019; 114:107–115.
6. Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population based study. Intest Res. 2019; 17:54–62.
7. Singh P, Ananthakrishnan A, Ahuja V. Pivot to Asia: inflammatory bowel disease burden. Intest Res. 2017; 15:138–141.
8. Hong SW, Ye BD. The first step to unveil the epidemiology of inflammatory bowel disease in Central Asia. Intest Res. 2020; 18:345–346.
9. Lui RN, Ng SC. The same intestinal inflammatory disease despite different genetic risk factors in the East and West? Inflamm Intest Dis. 2016; 1:78–84.

10. Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013; 62:630–649.

11. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280.

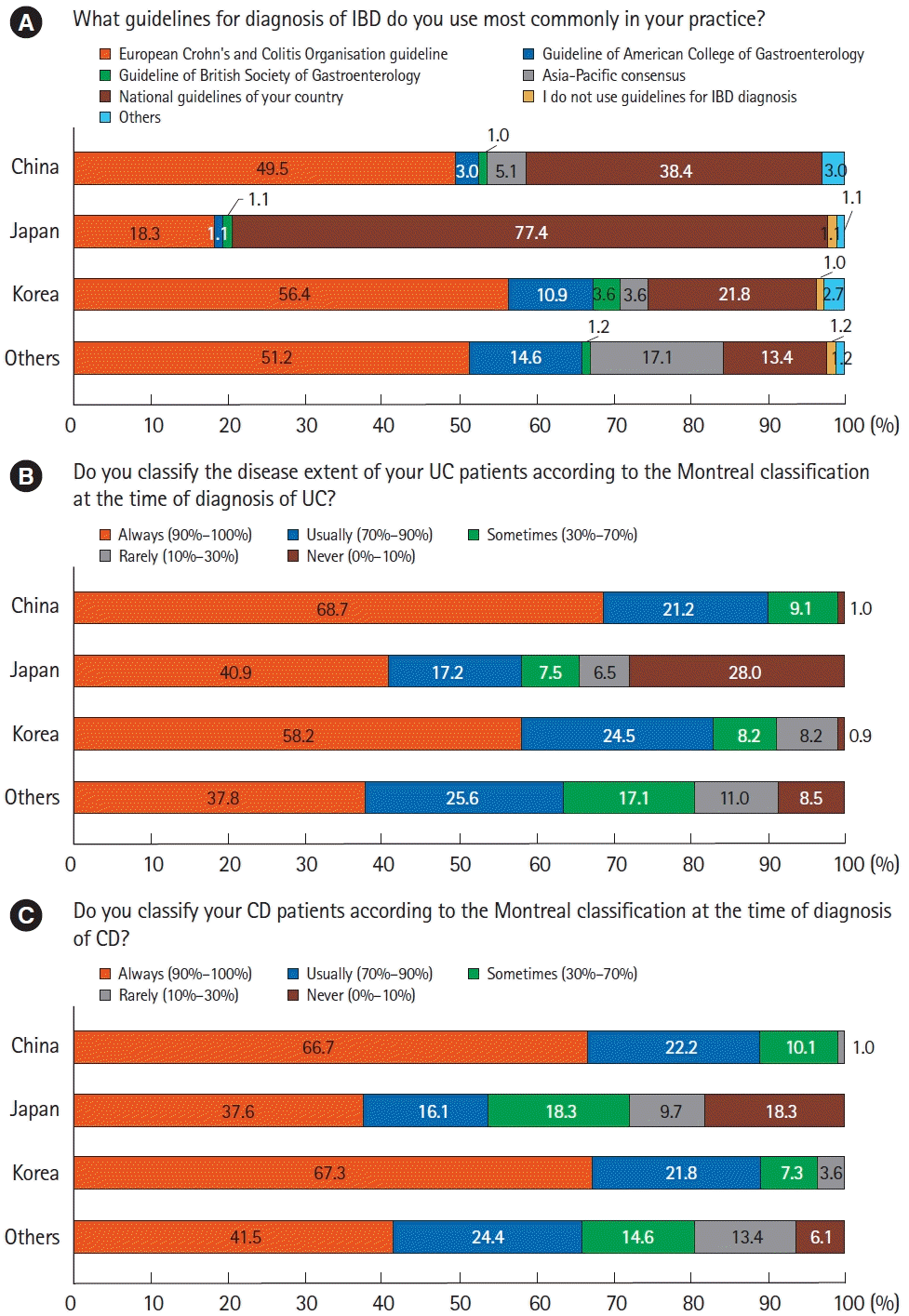
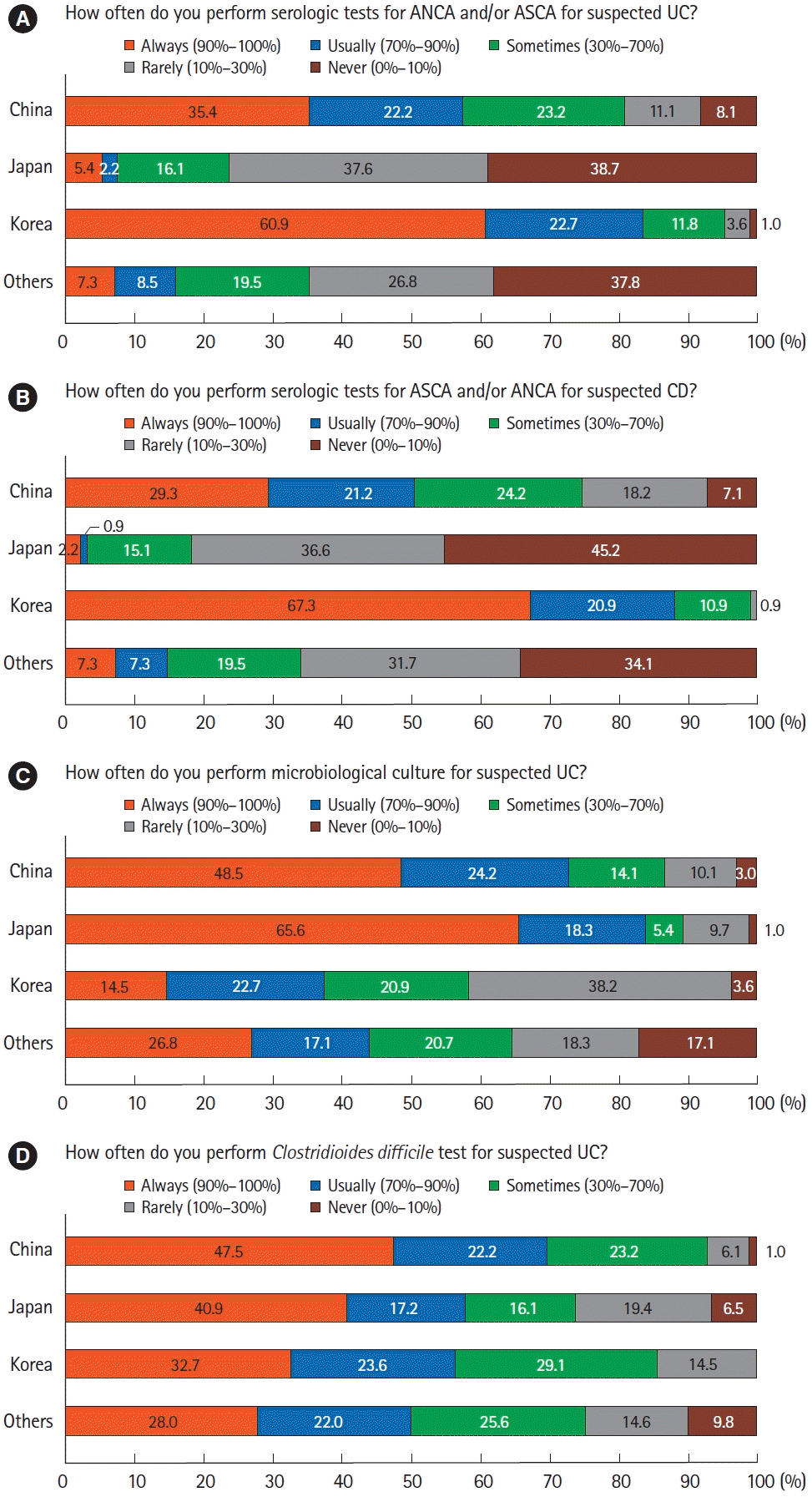
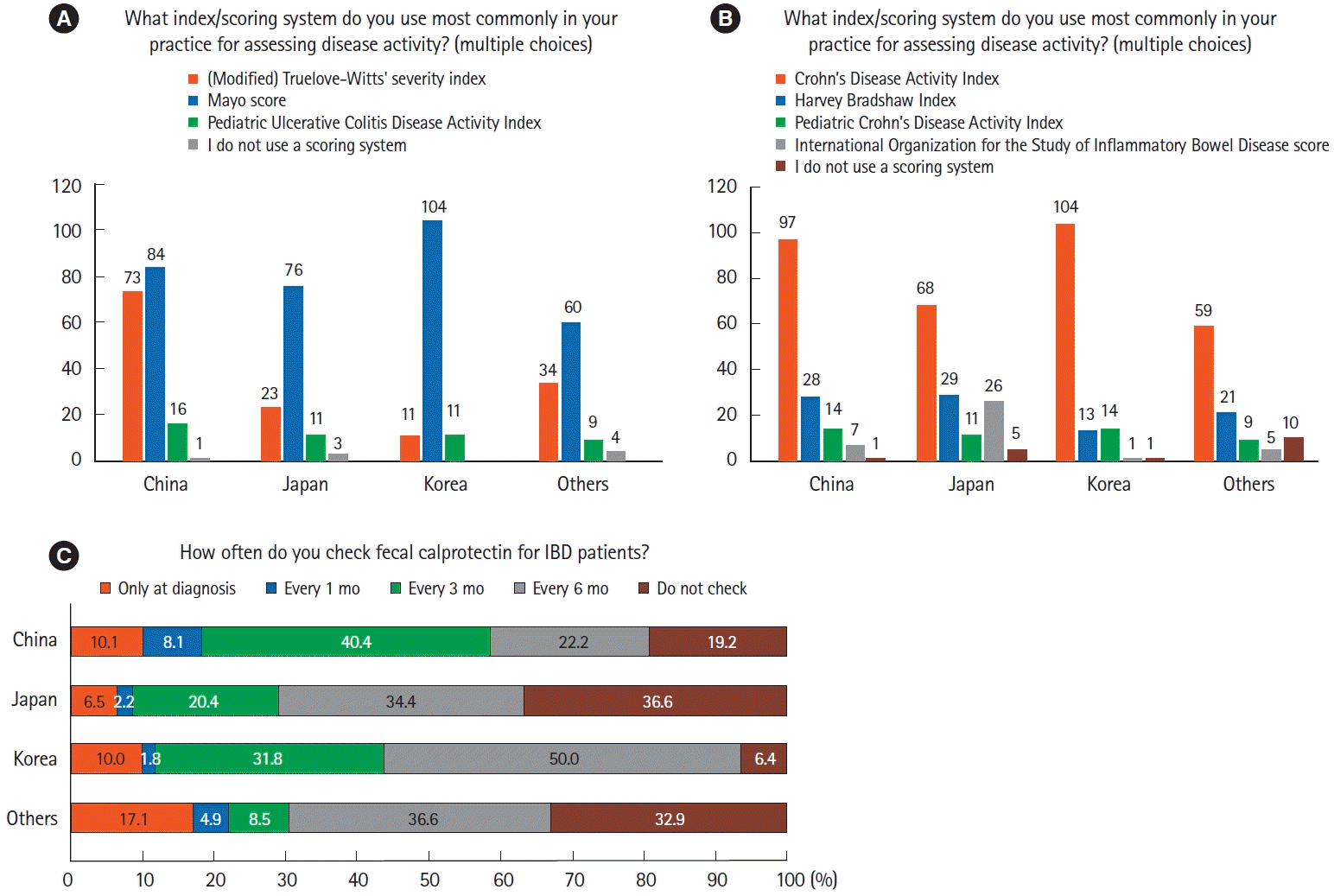
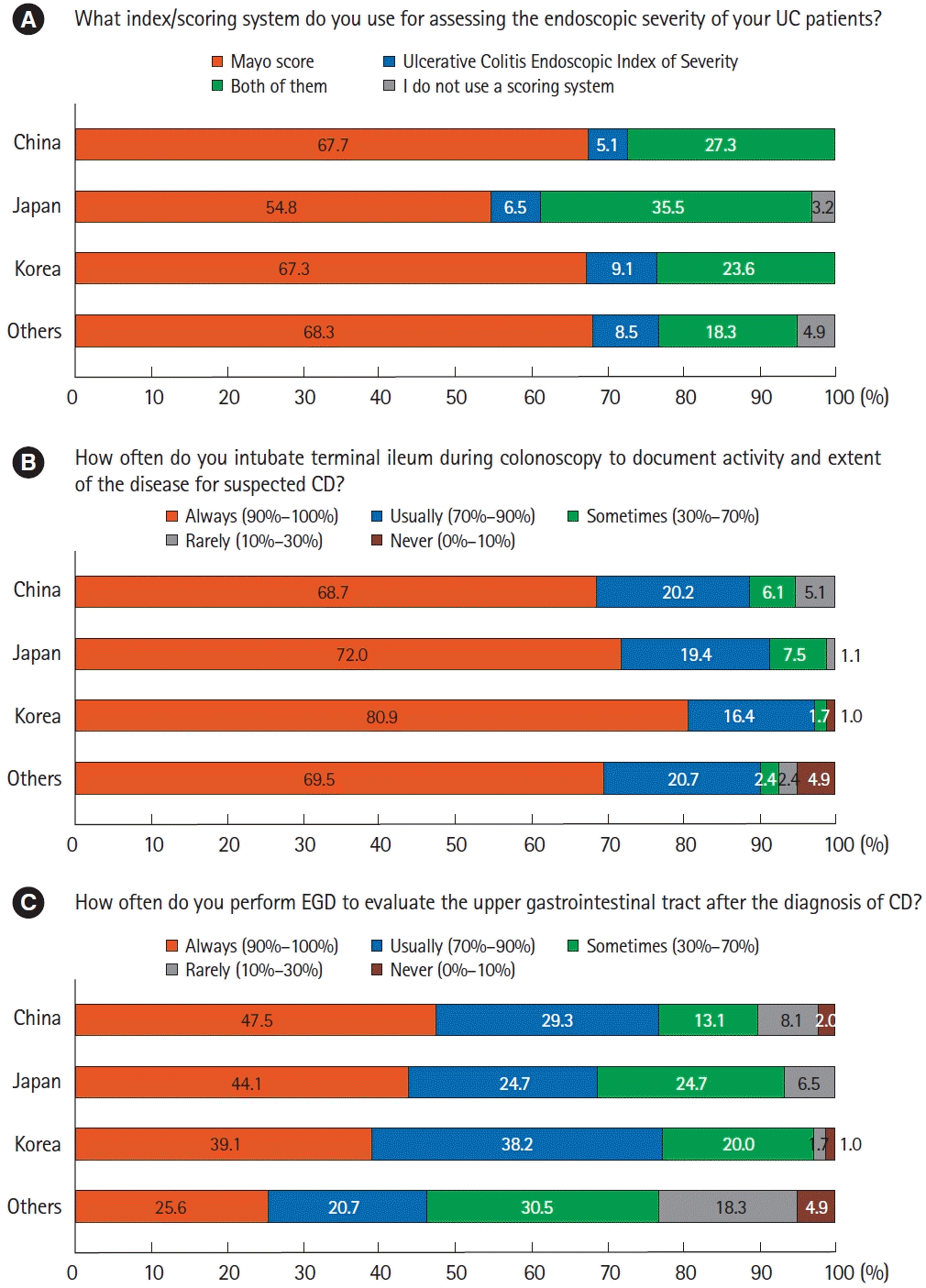
12. Kim ES, Chen M, Lee J, Lee CK, Kim YS. Diagnosis of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2nd Asian Organization for Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:224–230.

13. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.

14. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.

15. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.

16. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.

17. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018; 113:481–517.

18. Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021; 36:637–645.

19. Ng SC. Emerging leadership lecture: inflammatory bowel disease in Asia: emergence of a “Western” disease. J Gastroenterol Hepatol. 2015; 30:440–445.

20. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.
21. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.

22. von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007; 102:803–813.

23. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009; 15:1851–1858.
24. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.

25. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013; 145:987–995.

26. Ikeya K, Hanai H, Sugimoto K, et al. The ulcerative colitis endoscopic index of severity more accurately reflects clinical outcomes and long-term prognosis than the Mayo endoscopic score. J Crohns Colitis. 2016; 10:286–295.
27. Saigusa K, Matsuoka K, Sugimoto S, et al. Ulcerative colitis endoscopic index of severity is associated with long-term prognosis in ulcerative colitis patients treated with infliximab. Dig Endosc. 2016; 28:665–670.

28. Takenaka K, Kitazume Y, Fujii T, Tsuchiya K, Watanabe M, Ohtsuka K. Objective evaluation for treat to target in Crohn’s disease. J Gastroenterol. 2020; 55:579–587.
29. Lawrance IC, Murray K, Hall A, Sung JJ, Leong R. A prospective comparative study of ASCA and pANCA in Chinese and Caucasian IBD patients. Am J Gastroenterol. 2004; 99:2186–2194.

30. Kim YS, Kim YH, Kim WH, et al. Diagnostic utility of anti-Saccharomyces cerevisiae antibody (ASCA) and interferon-γ assay in the differential diagnosis of Crohn’s disease and intestinal tuberculosis. Clin Chim Acta. 2011; 412:1527–1532.

31. Ng SC, Hirai HW, Tsoi KK, et al. Systematic review with meta-analysis: accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J Gastroenterol Hepatol. 2014; 29:1664–1670.

32. Ghoshal UC, Ghoshal U, Singh H, Tiwari S. Anti-Saccharomyces cerevisiae antibody is not useful to differentiate between Crohn’s disease and intestinal tuberculosis in India. J Postgrad Med. 2007; 53:166–170.

33. Makharia GK, Sachdev V, Gupta R, Lal S, Pandey RM. Anti-Saccharomyces cerevisiae antibody does not differentiate between Crohn’s disease and intestinal tuberculosis. Dig Dis Sci. 2007; 52:33–39.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download