1. Andersen LF, Jacobs DR Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am J Clin Nutr. 2006; 83:1039–1046.

2. Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005; 129:1928–1936.

3. Modi AA, Feld JJ, Park Y, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010; 51:201–209.

4. Yang H, Rouse J, Lukes L, et al. Caffeine suppresses metastasis in a transgenic mouse model: a prototype molecule for prophylaxis of metastasis. Clin Exp Metastasis. 2004; 21:719–735.

5. Lu YP, Lou YR, Xie JG, et al. Caffeine and caffeine sodium benzoate have a sunscreen effect, enhance UVB-induced apoptosis, and inhibit UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2007; 28:199–206.

6. Alao JP, Sunnerhagen P. The ATM and ATR inhibitors CGK733 and caffeine suppress cyclin D1 levels and inhibit cell proliferation. Radiat Oncol. 2009; 4:51.

7. Kang SS, Han KS, Ku BM, et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010; 70:1173–1183.

8. Saiki S, Sasazawa Y, Imamichi Y, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011; 7:176–187.

9. Bessler H, Salman H, Bergman M, Djaldetti M. Caffeine alters cytokine secretion by PBMC induced by colon cancer cells. Cancer Invest. 2012; 30:87–91.

10. Bakuradze T, Lang R, Hofmann T, et al. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol Nutr Food Res. 2010; 54:1734–1743.

11. Jaquet M, Rochat I, Moulin J, Cavin C, Bibiloni R. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol. 2009; 130:117–121.

12. Kim JY, Kim DH, Jeong HG. Inhibitory effect of the coffee diterpene kahweol on carrageenan-induced inflammation in rats. Biofactors. 2006; 26:17–28.

13. Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015; 64:1063–1071.

14. Iriondo-DeHond A, Uranga JA, Del Castillo MD, Abalo R. Effects of coffee and its components on the gastrointestinal tract and the brain-gut axis. Nutrients. 2020; 13:88.

15. Nehlig A. Effects of coffee on the gastro-intestinal tract: a narrative review and literature update. Nutrients. 2022; 14:399.

16. Yang Y, Xiang L, He J. Beverage intake and risk of Crohn disease: a meta-analysis of 16 epidemiological studies. Medicine (Baltimore). 2019; 98:e15795.
17. Michels KB, Willett WC, Fuchs CS, Giovannucci E. Coffee, tea, and caffeine consumption and incidence of colon and rectal cancer. J Natl Cancer Inst. 2005; 97:282–292.

18. Wang R, Dashwood WM, Löhr CV, et al. Protective versus promotional effects of white tea and caffeine on PhIP-induced tumorigenesis and beta-catenin expression in the rat. Carcinogenesis. 2008; 29:834–839.

19. Bae JM, Shim SR. Coffee consumption and pancreatic cancer risk: a meta-epidemiological study of population-based cohort studies. Asian Pac J Cancer Prev. 2020; 21:2793–2798.

20. Um CY, McCullough ML, Guinter MA, Campbell PT, Jacobs EJ, Gapstur SM. Coffee consumption and risk of colorectal cancer in the Cancer Prevention Study-II Nutrition Cohort. Cancer Epidemiol. 2020; 67:101730.

21. Mackintosh C, Yuan C, Ou FS, et al. Association of coffee intake with survival in patients with advanced or metastatic colorectal cancer. JAMA Oncol. 2020; 6:1713–1721.

22. Lee IA, Kamba A, Low D, Mizoguchi E. Novel methylxanthine derivative-mediated anti-inflammatory effects in inflammatory bowel disease. World J Gastroenterol. 2014; 20:1127–1138.

23. Rao FV, Andersen OA, Vora KA, Demartino JA, van Aalten DM. Methylxanthine drugs are chitinase inhibitors: investigation of inhibition and binding modes. Chem Biol. 2005; 12:973–980.

24. Schüttelkopf AW, Andersen OA, Rao FV, et al. Screening-based discovery and structural dissection of a novel family 18 chitinase inhibitor. J Biol Chem. 2006; 281:27278–27285.

25. Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.

26. Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007; 56:21–27.

27. Mazur M, Zielińska A, Grzybowski MM, Olczak J, Fichna J. Chitinases and chitinase-like proteins as therapeutic targets in inflammatory diseases, with a special focus on inflammatory bowel diseases. Int J Mol Sci. 2021; 22:6966.

28. Ober C, Chupp GL. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009; 9:401–408.

29. Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009; 15:5249–5259.

30. Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003; 20:1–30.

31. Singh N, Shreshtha AK, Thakur MS, Patra S. Xanthine scaffold: scope and potential in drug development. Heliyon. 2018; 4:e00829.

32. Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015; 13:71–88.

33. Varani K, Portaluppi F, Merighi S, Ongini E, Belardinelli L, Borea PA. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation. 1999; 99:2499–2502.

34. Lazarus M, Shen HY, Cherasse Y, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011; 31:10067–10075.

35. Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999; 51:83–133.
36. Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006; 147(Suppl 1):S252–S257.

37. Guo Y, Zhang Z, Wu HE, Luo ZD, Hogan QH, Pan B. Increased thrombospondin-4 after nerve injury mediates disruption of intracellular calcium signaling in primary sensory neurons. Neuropharmacology. 2017; 117:292–304.

38. Roca DJ, Schiller GD, Farb DH. Chronic caffeine or theophylline exposure reduces gamma-aminobutyric acid/benzodiazepine receptor site interactions. Mol Pharmacol. 1988; 33:481–485.
39. Mattioli AV, Farinetti A. Espresso coffee, caffeine and colon cancer. World J Gastrointest Oncol. 2020; 12:601–603.

40. Lovallo WR, Farag NH, Vincent AS, Thomas TL, Wilson MF. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol Biochem Behav. 2006; 83:441–447.

41. Reis CEG, Dórea JG, da Costa THM. Effects of coffee consumption on glucose metabolism: a systematic review of clinical trials. J Tradit Complement Med. 2018; 9:184–191.

42. O’Keefe JH, DiNicolantonio JJ, Lavie CJ. Coffee for cardioprotection and longevity. Prog Cardiovasc Dis. 2018; 61:38–42.

43. Moisey LL, Kacker S, Bickerton AC, Robinson LE, Graham TE. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr. 2008; 87:1254–1261.

44. Gabbert C, König IR, Lüth T, et al. Coffee, smoking and aspirin are associated with age at onset in idiopathic Parkinson’s disease. J Neurol. 2022; 269:4195–4203.

45. Ma JY, Li RH, Huang K, Tan G, Li C, Zhi FC. Increased expression and possible role of chitinase 3-like-1 in a colitis-associated carcinoma model. World J Gastroenterol. 2014; 20:15736–15744.

46. Nieber K. The impact of coffee on health. Planta Med. 2017; 83:1256–1263.

47. Hu Y, Ding M, Yuan C, et al. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology. 2018; 154:916–926.

48. Sinha R, Cross AJ, Daniel CR, et al. Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. Am J Clin Nutr. 2012; 96:374–381.

49. Shojaei-Zarghani S, Yari Khosroushahi A, Rafraf M, Asghari-Jafarabadi M, Azami-Aghdash S. Dietary natural methylxanthines and colorectal cancer: a systematic review and meta-analysis. Food Funct. 2020; 11:10290–10305.

50. van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med. 2020; 383:369–378.

51. Sewter R, Heaney S, Patterson A. Coffee consumption and the progression of NAFLD: a systematic review. Nutrients. 2021; 13:2381.

52. Ebadi M, Ip S, Bhanji RA, Montano-Loza AJ. Effect of coffee consumption on non-alcoholic fatty liver disease incidence, prevalence and risk of significant liver fibrosis: systematic review with meta-analysis of observational studies. Nutrients. 2021; 13:3042.

53. Desmond PV, Patwardhan RV, Johnson RF, Schenker S. Impaired elimination of caffeine in cirrhosis. Dig Dis Sci. 1980; 25:193–197.

54. Leung WW, Ho SC, Chan HL, Wong V, Yeo W, Mok TS. Moderate coffee consumption reduces the risk of hepatocellular carcinoma in hepatitis B chronic carriers: a case-control study. J Epidemiol Community Health. 2011; 65:556–558.

55. Batista MN, Carneiro BM, Braga AC, Rahal P. Caffeine inhibits hepatitis C virus replication in vitro. Arch Virol. 2015; 160:399–407.

56. Shan L, Wang F, Zhai D, Meng X, Liu J, Lv X. Caffeine in liver diseases: pharmacology and toxicology. Front Pharmacol. 2022; 13:1030173.

57. Torres DM, Harrison SA. Is it time to write a prescription for coffee? Coffee and liver disease. Gastroenterology. 2013; 144:670–672.

58. Anty R, Marjoux S, Iannelli A, et al. Regular coffee but not espresso drinking is protective against fibrosis in a cohort mainly composed of morbidly obese European women with NAFLD undergoing bariatric surgery. J Hepatol. 2012; 57:1090–1096.

59. Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008; 48:993–999.

60. Paiva C, Beserra B, Reis C, Dorea JG, Da Costa T, Amato AA. Consumption of coffee or caffeine and serum concentration of inflammatory markers: a systematic review. Crit Rev Food Sci Nutr. 2019; 59:652–663.

61. Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012; 366:1891–1904.

62. Rodas L, Riera-Sampol A, Aguilo A, Martínez S, Tauler P. Effects of habitual caffeine intake, physical activity levels, and sedentary behavior on the inflammatory status in a healthy population. Nutrients. 2020; 12:2325.

63. Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006; 130:398–411.

64. Tang H, Fang Z, Sun Y, et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur Respir J. 2010; 35:757–760.

65. Shan Z, Liu X, Chen Y, et al. Chitinase 3-like-1 promotes intrahepatic activation of coagulation through induction of tissue factor in mice. Hepatology. 2018; 67:2384–2396.

66. Matsumoto T, Tsurumoto T. Serum YKL-40 levels in rheumatoid arthritis: correlations between clinical and laborarory parameters. Clin Exp Rheumatol. 2001; 19:655–660.
67. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. 2020; 5:201.

68. Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010; 8:564–577.

69. Uniken Venema WT, Voskuil MD, Dijkstra G, Weersma RK, Festen EA. The genetic background of inflammatory bowel disease: from correlation to causality. J Pathol. 2017; 241:146–158.

70. Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011; 140:268–275.

71. Dahan S, Roda G, Pinn D, et al. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008; 134:192–203.

72. Huang XL, Xu J, Zhang XH, et al. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm Res. 2011; 60:727–734.

73. Managlia E, Katzman RB, Brown JB, Barrett TA. Antioxidant properties of mesalamine in colitis inhibit phosphoinositide 3-kinase signaling in progenitor cells. Inflamm Bowel Dis. 2013; 19:2051–2060.

74. Lee IA, Low D, Kamba A, Llado V, Mizoguchi E. Oral caffeine administration ameliorates acute colitis by suppressing chitinase 3-like 1 expression in intestinal epithelial cells. J Gastroenterol. 2014; 49:1206–1216.

75. Ghasemi-Pirbaluti M, Motaghi E, Najafi A, Hosseini MJ. The effect of theophylline on acetic acid induced ulcerative colitis in rats. Biomed Pharmacother. 2017; 90:153–159.

76. Karatay E, Gül Utku Ö, Erdal H, et al. Pentoxifylline attenuates mucosal damage in an experimental model of rat colitis by modulating tissue biomarkers of inflammation, oxidative stress, and fibrosis. Turk J Med Sci. 2017; 47:348–356.

77. Barthel C, Wiegand S, Scharl S, et al. Patients’ perceptions on the impact of coffee consumption in inflammatory bowel disease: friend or foe? A patient survey. Nutr J. 2015; 14:78.
78. Georgiou AN, Ntritsos G, Papadimitriou N, Dimou N, Evangelou E. Cigarette smoking, coffee consumption, alcohol intake, and risk of Crohn’s disease and ulcerative colitis: a Mendelian randomization study. Inflamm Bowel Dis. 2021; 27:162–168.

79. Nakamura T, Ishikawa H, Mutoh M, et al. Coffee prevents proximal colorectal adenomas in Japanese men: a prospective cohort study. Eur J Cancer Prev. 2016; 25:388–394.

80. Roudi F, Khayyatzadeh SS, Ghazizadeh H, et al. The relationship between dietary intakes and prevalence of irritable bowel syndrome in adolescent girls: a cross-sectional study. Indian J Gastroenterol. 2021; 40:220–226.

81. Li M, Zhang R, Xin M, et al. Discovery and validation of potential serum biomarkers with pro-inflammatory and DNA damage activities in ulcerative colitis: a comprehensive untargeted metabolomic study. Metabolites. 2022; 12:997.

82. Niewiadomski O, Studd C, Wilson J, et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016; 46:669–676.

83. Burisch J, Pedersen N, Cukovic-Cavka S, et al. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe: an ECCO-EpiCom study. J Crohns Colitis. 2014; 8:607–616.

84. Brown AC, Rampertab SD, Mullin GE. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2011; 5:411–425.

85. Korzenik JR; NDSG. Diverticulitis: new frontiers for an old country: risk factors and pathogenesis. J Clin Gastroenterol. 2008; 42:1128–1129.
86. Bott C, Rudolph MW, Schneider AR, et al. In vivo evaluation of a novel pH- and time-based multiunit colonic drug delivery system. Aliment Pharmacol Ther. 2004; 20:347–353.

87. Zhang X, Huang H, Sun S, et al. Induction of apoptosis via inactivating PI3K/AKT pathway in colorectal cancer cells using aged Chinese Hakka stir-fried green tea extract. Molecules. 2022; 27:8272.

88. Bartolomeu AR, Romualdo GR, Lisón CG, et al. Caffeine and chlorogenic acid combination attenuate early-stage chemically induced colon carcinogenesis in mice: involvement of oncomiR miR-21a-5p. Int J Mol Sci. 2022; 23:6292.

89. Lu YT, Gunathilake M, Lee J, et al. Coffee consumption and its interaction with the genetic variant AhR rs2066853 in colorectal cancer risk: a case-control study in Korea. Carcinogenesis. 2022; 43:203–216.

90. Castaldo L, Izzo L, Narváez A, Rodríguez-Carrasco Y, Grosso M, Ritieni A. Colon bioaccessibility under in vitro gastrointestinal digestion of different coffee brews chemically profiled through UHPLC-Q-Orbitrap HRMS. Foods. 2021; 10:179.

91. Bai B, Shan L, Wang J, et al. Small molecule 2,3-DCPE induces S phase arrest by activating the ATM/ATR-Chk1-Cdc25A signaling pathway in DLD-1 colon cancer cells. Oncol Lett. 2020; 20:294.

92. Chapelle N, Martel M, Toes-Zoutendijk E, Barkun AN, Bardou M. Recent advances in clinical practice: colorectal cancer chemoprevention in the average-risk population. Gut. 2020; 69:2244–2255.

93. Gao X, Li X, Ho CT, et al. Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling pathway. Food Res Int. 2020; 129:108854.
94. El-Far AH, Darwish NH, Mousa SA. Senescent colon and breast cancer cells induced by doxorubicin exhibit enhanced sensitivity to curcumin, caffeine, and thymoquinone. Integr Cancer Ther. 2020; 19:1534735419901160.
95. Sartini M, Bragazzi NL, Spagnolo AM, et al. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2019; 11:694.
96. Soares PV, Kannen V, Jordão Junior AA, Garcia SB. Coffee, but neither decaffeinated coffee nor caffeine, elicits chemoprotection against a direct carcinogen in the colon of Wistar rats. Nutr Cancer. 2019; 71:615–623.
97. Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. 2017; 37:131–156.

98. Nakayama T, Funakoshi-Tago M, Tamura H. Coffee reduces KRAS expression in Caco-2 human colon carcinoma cells via regulation of miRNAs. Oncol Lett. 2017; 14:1109–1114.

99. Guercio BJ, Sato K, Niedzwiecki D, et al. Coffee intake, recurrence, and mortality in stage III colon cancer: results from CALGB 89803 (Alliance). J Clin Oncol. 2015; 33:3598–3607.

100. Choi DW, Lim MS, Lee JW, et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol Ther (Seoul). 2015; 23:128–133.

101. Isshiki M, Ohta H, Tamura H. Coffee reduces SULT1E1 expression in human colon carcinoma Caco-2 cells. Biol Pharm Bull. 2013; 36:299–304.
102. Vitaglione P, Fogliano V, Pellegrini N. Coffee, colon function and colorectal cancer. Food Funct. 2012; 3:916–922.

103. Nagatomo K, Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A. 2008; 105:17373–17378.

104. Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019; 363:1345–1349.

105. Jacobsen H, Poulsen M, Dragsted LO, Ravn-Haren G, Meyer O, Lindecrona RH. Carbohydrate digestibility predicts colon carcinogenesis in azoxymethane-treated rats. Nutr Cancer. 2006; 55:163–170.

106. Wang B, Bobe G, LaPres JJ, Bourquin LD. Dietary carbohydrate source alters gene expression profile of intestinal epithelium in mice. Nutr Cancer. 2009; 61:146–155.

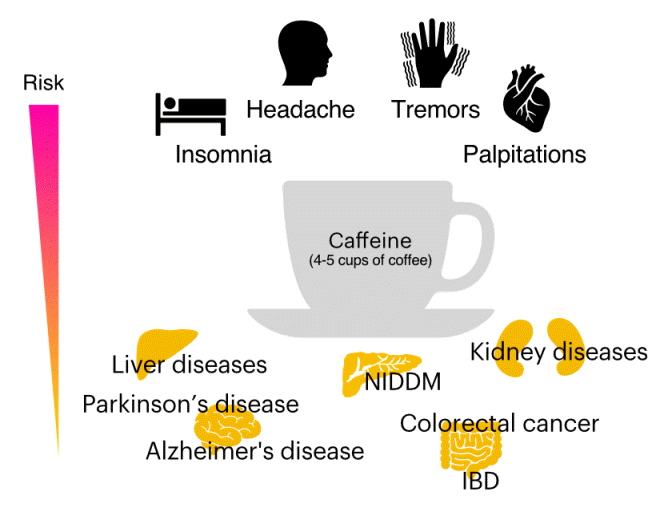




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download