Abstract
Background/Aims
Increasing resistance to clarithromycin (CAM) of Helicobacter pylori (H. pylori) is one of the main causes of recent decrease in eradication rate of standard triple therapy. The aim of this study was to evaluate the usefulness of 7-day tailored therapy based on the existence of CAM resistance.
Methods
From January 2017 to May 2022, a total of 481 consecutive patients with H. pylori infection were recruited in Daegu Catholic University Medical Center. Treatment regimen was selected based on the result of CAM resistance test. Patients with CAM resistance (R group) were treated with bismuth-based quadruple therapy for 7 days. Patients without CAM resistance (S group) were treated with standard triple therapy for 7 days.
Results
The overall H. pylori eradication rate was 89.4% (379 of 424) by per-protocol (PP) analysis. Patients with CAM resistance mutation included 166 patients (34.5%). The eradication rates of each group were 88.8% (135 of 152) and 89.7% (244 of 272) by PP analysis, for R and S group respectively. By intention-to-treat (ITT) analysis, the eradication rates were 81.3% (135 of 166) and 77.5% (244 of 315) for R and S group. CAM resistance was identified with a dual-priming oligonucleotide-based multiplex PCR.
Helicobacter pylori (H. pylori) infection is known to be main pathogen of chronic gastritis, peptic ulcer disease, gastric mucosa- associated lymphoid tissue lymphoma, and gastric adenocarcinoma.1-4 Also, there are studies showing that H. pylori eradication treatment can prevent gastric cancer.3,4 In this situation, successful H. pylori eradication is an important issue in South Korea having high incidence of gastric cancer. But the eradication rate with empirical standard triple therapy (STT) has gradually decreased recently and has fallen to almost 70%, which is an unacceptable level.5-8 Clarithromycin (CAM) resistance is a main factor of the treatment failure in the H. pylori infection.9-11
The resistance rate against CAM was 17.8% in a nationwide study of antibiotic resistance mapping in Korea.9 According to Maastricht V guideline of Europe, it is recommended not to use CAM based triple therapy without antibiotic susceptibility test where drug resistance is over 15%.1 Although tailored eradication therapy according to the antibiotic susceptibility test would be ideal, culturing H. pylori is difficult and time-consuming, so there is a limit to its application to treatment.12 Instead, biomolecular methods which can confirm CAM resistance, such as dual-priming oligonucleotide (DPO)-based multiplex polymerase chain reaction (PCR) assay, have been developed and applied at treatment recently.13-15
We aimed to evaluate the efficacy of 7-day tailored therapy based on the DPO-based multiplex PCR. The secondary aim was to evaluate whether 7-day bismuth-based quadruple therapy (BQT) is effective or not as first line treatment regimen of H. pylori eradication in patients with CAM resistance.
A total of 547 H. pylori infected patients who received eradication treatment after clarithromycin resistance test were recruited in Daegu Catholic University Medical Center from January 2017 to May 2022. Of them, a total of 481 patients who received 7-day tailored therapy based on the results of clarithromycin resistance were included in this study. We collected extra group who received treatment other than 7-day tailored therapy after clarithromycin resistance test (n=66) (Fig. 1).
Exclusion criteria were as follows; 1) previous eradication history of H. pylori within 1 year, 2) history of gastric surgery, 3) history of side effect or allergy to study drugs, 4) pregnancy or breast-feeding, 5) severe comorbidities like end stage renal disease or liver cirrhosis. The present study protocol was reviewed and approved by the Institutional Review Board of Daegu Catholic University Medical Center (approval No. CR-22-198). Because this study was conducted through a retrospective analysis of existing clinical data, the need for informed consent from patients was waived.
Active H. pylori infection was determined by rapid urease testing (Kimberly-Clark, Roswell, GA, USA). Patients underwent esophagogastroduodenoscopy, and single biopsy specimen was obtained from the antrum or body of stomach, respectively. All rapid urease tests were monitored for any color change for up to 24 hours. Positive results for H. pylori were considered when the color of the kits changed from light orange into pink/red.
Biopsy specimens for rapid urease test were used to detect CAM resistance-related point mutations. DNA was extracted from biopsy specimens and DPO-based multiplex PCR was performed using a Seeplex® H. pylori-ClaR ACE Detection kit (Seegene Inc., Seoul, Korea). Point mutations were identified by PCR amplification of a portion of the 23S ribosomal RNA gene. After gel electrophoresis and ultraviolet transillumination were performed, the amplified DNA products were determined to have the A2142G or A2143G point mutation, respectively when a 194-bp band or a 475-bp band was detected.
The flow diagram of this study is shown in Fig. 1. Participants were recommended to undergo tailored H. pylori eradication treatment based on the result of DPO-PCR. The patients were divided into two groups based on the existence of CAM resistance. Patients without CAM resistance mutation (S group) were treated with STT (standard dose of proton pump inhibitor [PPI] twice a day, amoxicillin 1,000 mg twice a day and CAM 500 mg twice a day) for 7 days. Patients with CAM resistance mutation (R group) were treated with BQT (standard dose of PPI twice a day, bismuth 300 mg four times a day, metronidazole 500 mg three times a day and tetracycline 500 mg four times a day) for 7 days.
To confirm eradication of H. pylori, rapid urease test or 13C-urea breath test was performed 4 to 6 weeks after the completion of treatment. The 13C-urea breath test was performed after discontinuation of drug (such as antibiotics and PPI) for 4 weeks and fasting for at least 4 hours prior to the test. Breath samples were obtained before and 20 minutes after taking the 100 mg tablet of 13C-urea (UBiTkit™; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan). Collected breath samples were analyzed using an isotope-selective, nondispersive infrared spectrometer (UBiT-IR 300®; Otsuka Pharmaceutical Co. Ltd.). Successful eradication was defined as negative result of rapid urease test or a delta 13CO2 below 2.5‰. Drug compliance was considered as poor if drug intake was less than 80% of the total medication prescribed. We excluded patients from per-protocol (PP) analysis who showed poor compliance or didn’t visit for the test to confirm eradication. Adverse events were defined as any undesirable medical symptoms or conditions that emerged in participants during drug administration, regardless of an apparent causal relationship.
The primary endpoint of this study was evaluating the efficacy of 7-day tailored therapy based on the DPO-based multiplex PCR. H. pylori eradication rates were analyzed based on the intention-to-treat (ITT) and PP analyses. Statistical differences in eradication rates among the different treatment regimens were assessed. Continuous variables and categorical variables were compared using the student’s t-tests and chi-square tests, respectively. A two-sided p-value less than 0.05 was considered statistically significant. All Statistical analyses were performed using IBM SPSS statistics for Windows version 26.0 (IBP Corp., Armonk, NY, USA).
Patient’s characteristics are shown in Table 1. 481 patients (306 males and 175 females) treated with H. pylori eradication were enrolled. They were distributed in the S group (n=315, 65.5%) and R group (n=166, 34.5%) (Table 1). Of the S group, 43 patients were excluded from PP analysis (41 follow-up loss, 2 poor compliance). Of the R group, 14 were excluded (13 follow-up loss, 1 poor compliance) (Fig. 1). Mean age of enrolled patients was 61.5±12.5 years (Table 1). Pathological diagnoses by endoscopy were as follows; adenoma (n=210, 43.7%) which was the most common, followed by peptic ulcer (n=182, 37.8%), adenocarcinoma (n=42, 8.7%), and others (n=47, 9.8%). Others included low-grade mucosa-associated lymphoid tissue (MALT) lymphoma, lymphofollicular gastritis, and acute H. pylori-related gastritis (Table 1). Lansoprazole was the most used PPI at eradication therapy (90.2%) (Table 1). Statistical differences were observed in the baseline characteristics of two groups. Proportion of male was higher in the S group (n=215, 68.3%) than in the R group (n=91, 54.8%) (p=0.004). Smoking rates were also higher in the S group (n=83, 30.5%) than in the R group (n=27, 17.8%) (p=0.004). Body mass index was lower in the S group (23.7 kg/m2) than in the R group (24.5 kg/m2) (p=0.021) (Table 1).
The overall eradication rate was 89.4% in PP and 78.8% in ITT analysis, respectively (Table 2). In PP analysis, eradication rates of R group and S group were 88.8% (135/152) and 89.7% (244/272), respectively (p=0.775) (Table 2). In ITT analysis, eradication rates of R group and S group were 81.3% (135/166) and 77.5% (244/315) (p=0.233) (Table 2). No serious adverse events were reported during treatment in either group. In the S group, there was one case of nausea and one case of headache. In the R group, there was one case of nausea with vomiting.
If treatment failure with 7-day tailored therapy was confirmed 4 to 6 weeks after treatment completion, the choice of second-line therapy and its specific treatment regimen were determined based to the clinician’s judgement. In R group, 17 patients experienced treatment failure with the first-line 7-day BQT. Among these patients, four received an additional 7 days of BQT, resulting in an eradication rate of 75.0% (3 out of 4 patients). Among the 28 patients who experienced treatment failure in S group, 14 patients received 7 days of BQT as second-line therapy, and 12 of them achieved successful eradication, eradication rate of 85.7%.
Of a total of 547 patients who underwent the clarithromycin resistance test, 66 patients (12.1%) received an eradication regimen other than the 7-day tailored therapy (Fig. 1). Among them, 23 patients received tailored therapy for 14 days. The overall eradication rate of them was 90.0% (18/20) in PP analysis and 78.3% (18/23) in ITT analysis, respectively (Table 3). The eradication rates of CAM-resistant patients who received 14-day BQT was 80.0% (4/5) in PP analysis, and 66.7% (4/6) in ITT analysis. In CAM-sensitive patients who received 14-day STT, the eradication rate was 93.3% (14/15) in PP analysis, and 82.4% (14/17) in ITT analysis. (Table 3).
166 patients (34.5%) showed point mutations associated with CAM resistance (Table 1). Among them, A2143G mutation was the most common point mutation (86.8%, 144/166). A2142G mutation was found in 19 patients (11.4%) (Table 4). Three patients had double mutations of A2143G and A2142G. We analyzed eradication rate according to the type of point mutation using ITT analysis and PP analysis (Table 4). The eradication rates of patients with A2142G mutation and A2143G mutation were 89.5% (17/19), 80.6% (116/144) in ITT analysis, and 94.4% (17/18), 88.5% (116/131) in PP analysis, respectively (Table 4). Patients with double mutations of A2142G and A2143G showed lower eradication rate (66.7%, 2/3) (Table 4).
Successful eradication of H. pylori is an important worldwide issue. However, the eradication rate of STT has recently decreased to nearly 70%, and CAM resistance of H. pylori is known to be the main cause of eradication failure.16-23 Many studies have suggested various tailored therapy based on antibiotic susceptibility test, as an alternative.16-23
Tailored therapies showed a higher success rate of eradication compared to empirical therapy, and there were studies with eradication rates exceeding 90%.16-20,23 Tailored therapy has the advantage of avoiding unnecessary abuse of antibiotics. Additionally, some studies have shown it to be more cost-effective than empirical therapy.21,22
In the updated clinical practice guidelines of Korea, tailored therapy of H. pylori eradication is recommended as 7-day STT in CAM sensitive patients, and 7-day metronidazole triple therapy or BQT in CAM resistant patients.24 However, there is controversy about which regimen should be used in CAM resistant H. pylori infection.
Many previous studies have shown poor outcomes of metronidazole- based therapy in CAM resistant patients.16,17,25 In a previous study, 14-day metronidazole triple therapy was used in the CAM resistant patients and showed low eradication rate (64.7% by ITT analysis, 70.5% by PP analysis).17 They assumed that the cause of the low eradication rate was the high rate of dual resistance to CAM and metronidazole.17 A Korean randomized controlled trial (782 patients) compared BQT and metronidazole intensified triple therapy for CAM resistant group. BQT showed significantly higher eradication rate than metronidazole- based therapy in PP analysis (95.1% vs. 76.4%, p=0.001).25
As above, BQT is highly effective for CAM resistant patients. But there are some limitations that patients had poor compliance and high rates of adverse events taking tetracycline and metronidazole together.26,27 To the best of our knowledge, there are two other published studies that have investigated tailored BQT with treatment durations of either 7 days or 14 days, and they have reported no significant differences in terms of eradication rates and incidence of adverse events.28,29 However, these studies have only been conducted with a small number of patients.
In this regard, we studied this 7-day tailored therapy to increase both success rate and compliance. We performed tailored therapy using 7-day STT or 7-day BQT depending on the existence of CAM resistance, with large samples, 481 patients. In our study, we achieved an overall high eradication rate of 89.4% according to PP analysis. Specifically, favorable eradication rates of 88.8% by PP analysis and 81.3% by ITT analysis were observed in CAM-resistant patients who underwent 7-day BQT regimen. Moreover, the incidence of adverse events was remarkably low, with 3 events reported among all patients and 1 event among those receiving BQT, indicating good treatment compliance. Considering the lack of consensus regarding the optimal duration of BQT for tailored treatment in patients with CAM resistance, our study results suggest the potential benefits and efficacy of a shorter treatment period of 7 days.
In this study, despite the limited number of patients available for comparison, we analyzed the eradication rate of patients who received 14-day tailored therapy. The overall eradication rates of these patients were 90.0% by PP analysis, demonstrating results similar to those observed with the 7-day tailored therapy. Additionally, CAM-resistant patients treated with 14-day BQT did not show better results than 7-day BQT with eradication rates of 80.0% by PP analysis and 66.7% by ITT analysis.
We also assessed the second-line treatment used in patients who experienced treatment failure with 7-day tailored therapy. Interestingly, we observed that when 7-day STT therapy failed in S group, an additional BQT therapy for only 7 days achieved successful eradication in 84% of patients. Although guidelines recommend 14-day BQT therapy as second- line therapy, despite the limited number of cases in our study, we can infer the potential success rate of 7-day BQT therapy as second-line treatment option.
The prevalence of CAM resistance in this study was 35%, which was higher than that of previous study. In a nationwide study of Korea published in 2019, the CAM resistance rate was 17.8%,9 and it showed a similar level in all regions including Gyeongsang-do which includes our center. As the cause of the high resistance rate in this study, it is possible that the resistance rate increased compared to 2019, and it is possible that there was a selection bias in patient recruitment as the study was conducted in a single tertiary medical center located in the city.
We used the DPO-PCR techniques using the specimen for rapid urease test to confirm the existence of CAM resistance, which let us quickly and easily get the results. However, we didn’t perform culture of H. pylori and antibiotic susceptibility test. Therefore, susceptibility tests to other antibiotics such as metronidazole and amoxicillin have not been studied. However, referring to previous study, molecular method showed satisfactory concordance with culture-based phenotypic methods.11,13,15,30 Considering studies showing that the A2143G, A2142G mutation were the most frequent, and that the A2143G mutation had the greatest influence on treatment failure, the DPO-PCR method used is in this study was seemed to be tolerable.14,31,32
As a result of the DPO-PCR, the A2143G mutation showed the highest proportion as 86.8% which was consistent with previous study.32 We analyzed success rate of eradication by types of mutation, and patients with double mutations of A2142G and A2143G showed lower eradication rate. However, the comparison was limited because the number of patients belonging to the A2142G group and the A2142G and A2143G double mutation group was insufficient. Further larger scale studies could be considered.
Our study has several limitations. First, since this study was conducted retrospectively at a single tertiary medical center in Korea, selection bias may occur in patient recruitment. However, our center performed clarithromycin resistance tests for most patients requiring eradication treatment during the study period. And, since the 7-day tailored therapy was not limited to a specific group, we expect the possibility of selection bias in choosing an eradication regimen to be low. Second, tailored therapy was not compared to other eradication regimens. However, there are many previous studies showing that tailored therapy has better outcomes than empirical therapy.16-20,23 The proportion of patients receiving regimens other than 7-day tailored therapy was small in our study, which limited the ability to compare each treatment group. To determine the appropriate treatment regimen or duration based on the presence of CAM resistance, further well-designed randomized controlled trials are necessary. Third, due to the retrospective study design, we did not investigate the patients’ previous antibiotic usage. And, we did not conduct the metronidazole resistance test, thus precluding confirmation of concurrent resistance to clarithromycin and metronidazole. Since concurrent resistance to both clarithromycin and metronidazole is an important factor in determining the appropriate duration of BQT, further research is necessary to address this aspect. However, our study has strong points that inclusion of a large number of patients and the performance of clarithromycin resistance tests. Furthermore, considering the lack of consensus regarding the optimal duration of BQT for first-line treatment in patients with CAM resistance, our study results suggest the potential benefits and efficacy of a shorter treatment period of 7 days.
In conclusion, in this study, 7-day tailored therapy based on the existence of CAM resistance showed a high eradication rate of 89.4% by PP analysis despite the high CAM resistance (34.5%). Also, 7-day BQT for CAM resistant patients showed high eradication rate and good compliance. The 7-day tailored therapy based on CAM resistance could be an acceptable treatment selection strategy for H. pylori eradication.
Notes
REFERENCES
1. Malfertheiner P, Megraud F, O'Morain CA, et al. 2017; Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 66:6–30. DOI: 10.1136/gutjnl-2016-312288. PMID: 27707777.
2. Fock KM, Graham DY, Malfertheiner P. 2013; Helicobacter pylori research: historical insights and future directions. Nat Rev Gastroenterol Hepatol. 10:495–500. DOI: 10.1038/nrgastro.2013.96. PMID: 23752823. PMCID: PMC3973742.
3. Shin SH, Jung DH, Kim JH, et al. 2015; Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS One. 10:e0143257. DOI: 10.1371/journal.pone.0143257. PMCID: PMC4651354.
4. Lee YC, Chiang TH, Chou CK, et al. 2016; Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology. 150:1113–1124.e5. DOI: 10.1053/j.gastro.2016.01.028. PMID: 26836587.
5. Shin WG, Lee SW, Baik GH, et al. 2016; Eradication rates of Helicobacter pylori in Korea over the past 10 years and correlation of the amount of antibiotics use: Nationwide survey. Helicobacter. 21:266–278. DOI: 10.1111/hel.12279. PMID: 26470999.
6. Kim BJ, Kim HS, Song HJ, et al. 2016; Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: Interim analysis. J Korean Med Sci. 31:1246–1253. DOI: 10.3346/jkms.2016.31.8.1246. PMID: 27478335. PMCID: PMC4951554.
7. Gong EJ, Yun SC, Jung HY, et al. 2014; Meta-analysis of first-line triple therapy for Helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 29:704–713. DOI: 10.3346/jkms.2014.29.5.704. PMID: 24851029. PMCID: PMC4024949.
8. Kim TH, Park JM, Cheung DY, Oh JH. 2020; Comparison of 7- and 14-day eradication therapy for Helicobacter pylori with first- and second-line regimen: Randomized clinical trial. J Korean Med Sci. 35:e33. DOI: 10.3346/jkms.2020.35.e33. PMID: 32030921. PMCID: PMC7008067.
9. Lee JH, Ahn JY, Choi KD, et al. 2019; Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 24:e12592. DOI: 10.1111/hel.12592. PMID: 31111572.
10. Park JY, Shin TS, Kim JH, Yoon HJ, Kim BJ, Kim JG. The prevalence of multidrug resistance of Helicobacter pylori and its impact on eradication in Korea from 2017 to 2019: A single-center study. Antibiotics (Basel). 2020; 9:646. DOI: 10.3390/antibiotics9100646. PMID: 32992624.
11. Kwon YH, Jeon SW, Nam SY, Lee HS, Park JH. 2019; Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: A single-center prospective pilot study. Helicobacter. 24:e12585. DOI: 10.1111/hel.12585. PMID: 30969459.
12. Zullo A, Hassan C, Lorenzetti R, Winn S, Morini S. 2003; A clinical practice viewpoint: to culture or not to culture Helicobacter pylori? Dig Liver Dis. 35:357–361. DOI: 10.1016/S1590-8658(03)00081-1. PMID: 12846409.
13. Owen RJ. 2002; Molecular testing for antibiotic resistance in Helicobacter pylori. Gut. 50:285–289. DOI: 10.1136/gut.50.3.285. PMID: 11839700. PMCID: PMC1773144.
14. De Francesco V, Margiotta M, Zullo A, et al. 2006; Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 144:94–100. DOI: 10.7326/0003-4819-144-2-200601170-00006. PMID: 16418408.
15. Oleastro M, Ménard A, Santos A, et al. 2003; Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 41:397–402. DOI: 10.1128/JCM.41.1.397-402.2003. PMID: 12517879. PMCID: PMC149634.
16. Seo SI, Do BJ, Kang JG, et al. 2019; Helicobacter pylori eradication according to sequencing-based 23S ribosomal RNA point mutation associated with clarithromycin resistance. J Clin Med. 9:54. DOI: 10.3390/jcm9010054. PMID: 31881688. PMCID: PMC7019680.
17. Ong S, Kim SE, Kim JH, et al. 2019; Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter. 24:e12654. DOI: 10.1111/hel.12654. PMID: 31411793.
18. Lee HJ, Kim JI, Cheung DY, et al. 2013; Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. 208:1123–1130. DOI: 10.1093/infdis/jit287. PMID: 23801607.
19. Pan J, Shi Z, Lin D, et al. 2020; Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial. Front Med. 14:43–50. DOI: 10.1007/s11684-019-0706-8. PMID: 31907860.
20. Choi YI, Chung JW, Park DK, et al. 2019; Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J Gastroenterol. 25:6743–6751. DOI: 10.3748/wjg.v25.i46.6743. PMID: 31857776.
21. Cho JH, Jeon SR, Kim HG, Jin SY, Park S. 2019; Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol. 34:700–706. DOI: 10.1111/jgh.14383. PMID: 30011083.
22. Gweon TG, Kim JS, Kim BW. 2018; An Economic modeling study of Helicobacter pylori eradication: Comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 12:648–654. DOI: 10.5009/gnl18079. PMCID: PMC6254616.
23. López-Góngora S, Puig I, Calvet X, et al. 2015; Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 70:2447–2455. DOI: 10.1093/jac/dkv155. PMID: 26078393.
24. Jung HK, Kang SJ, Lee YC, et al. 2021; Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J Intern Med. 36:807–838. DOI: 10.3904/kjim.2020.701. PMID: 34092054. PMCID: PMC8273819.
25. Seo SI, Lim H, Bang CS, et al. 2022; Bismuth-based quadruple therapy versus metronidazole-intensified triple therapy as a first-line treatment for clarithromycin-resistant Helicobacter pylori infection: A multicenter randomized controlled trial. Gut Liver. 16:697–705. DOI: 10.5009/gnl210365.
26. Liou JM, Fang YJ, Chen CC, et al. 2016; Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 388:2355–2365. DOI: 10.1016/S0140-6736(16)31409-X. PMID: 27769562.
27. Kim YI, Lee JY, Kim CG, Park B, Park JY, Choi IJ. 2021; Ten-day bismuth-containing quadruple therapy versus 7-day proton pump inhibitor-clarithromycin containing triple therapy as first-line empirical therapy for the Helicobacter pylori infection in Korea: a randomized open-label trial. BMC Gastroenterol. 21:95. DOI: 10.1186/s12876-021-01680-1. PMID: 33653284. PMCID: PMC7923489.
28. Na SY, Kim BW, Kim MJ, Choe Y, Kim JS. Effective eradication regimen and duration according to the clarithromycin susceptibility of helicobacter pylori determined using dual priming oligonucleotide-based multiplex polymerase chain reaction. Gut Liver. 2022; Sep. 28. doi: 10.5009/gnl220256. DOI: 10.5009/gnl220256.
29. Kim SY, Park JM, Lim CH, et al. 2021; Types of 23S Ribosomal RNA Point Mutations and Therapeutic Outcomes for Helicobacter pylori. Gut Liver. 15:528–536. DOI: 10.5009/gnl20225. PMID: 33376228. PMCID: PMC8283296.
30. Woo HY, Park DI, Park H, et al. 2009; Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. 14:22–28. DOI: 10.1111/j.1523-5378.2009.00654.x. PMID: 19191892.
31. Hwang TJ, Kim N, Kim HB, et al. 2010; Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 44:536–543. DOI: 10.1097/MCG.0b013e3181d04592. PMID: 20179610.
32. Park CG, Kim S, Lee EJ, Jeon HS, Han S. 2018; Clinical relevance of point mutations in the 23S rRNA gene in Helicobacter pylori eradication: A prospective, observational study. Medicine (Baltimore). 97:e11835. DOI: 10.1097/MD.0000000000011835. PMID: 30113472. PMCID: PMC6112885.
Fig. 1
Flow diagram of the study. S group was defined as a patient without CAM resistance, and R group was defined as a patient with CAM resistance. H. pylori, Helicobacter pylori; STT, standard triple therapy; BQT, bismuth-based quadruple therapy; ITT, intention-to-treat; PP, per-protocol; CAM, clarithromycin.
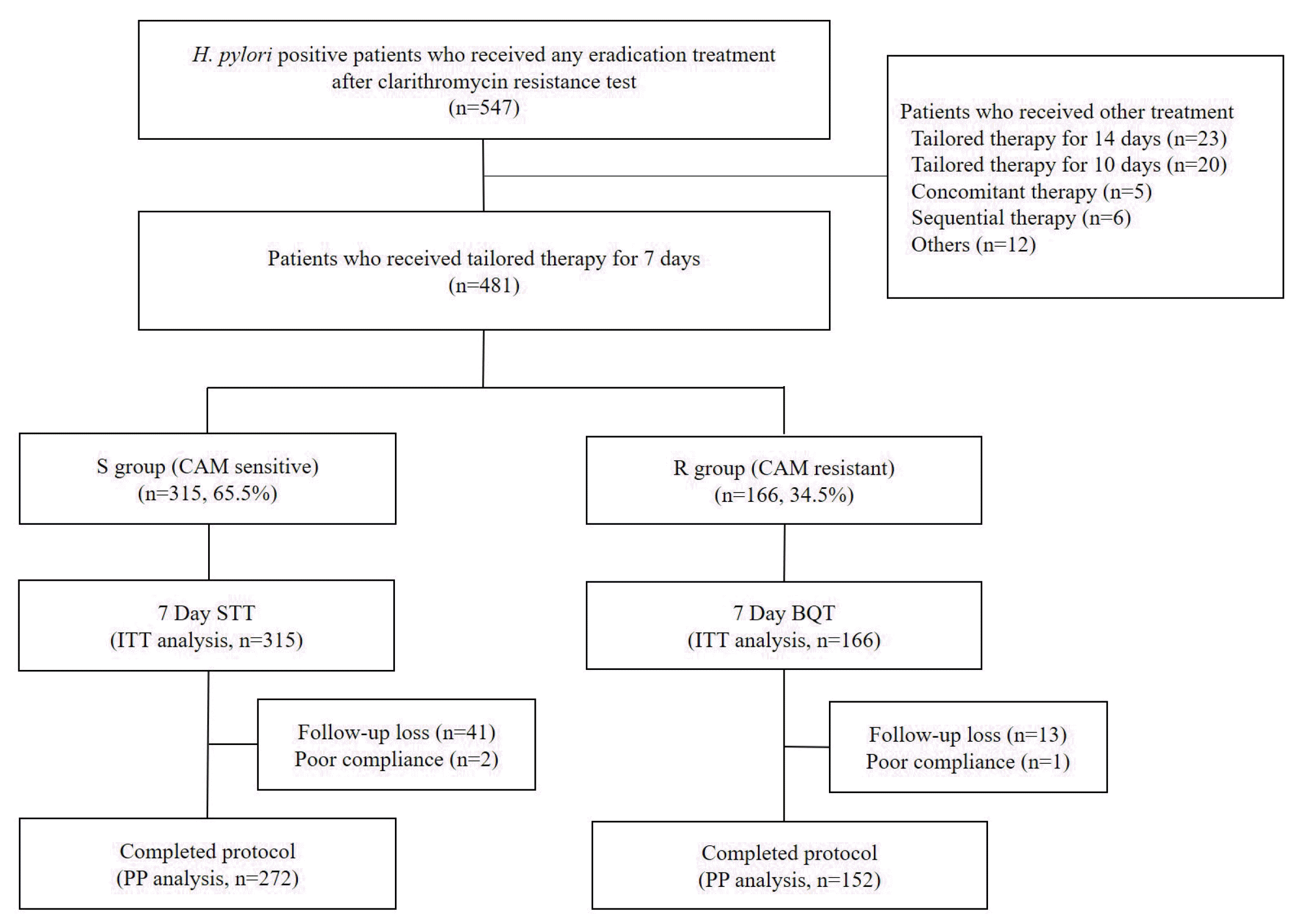
Table 1
Baseline Characteristics of the Enrolled Patients
| Variable | Total | S groupa | R groupa | p-valueb |
|---|---|---|---|---|
| Number | 481 (100) | 315 (65.5) | 166 (34.5) | |
| Age | 61.5±12.5 | 61.1±12.8 | 62.5±11.9 | 0.166 |
| Male | 306 (63.6) | 215 (68.3) | 91 (54.8) | 0.004 |
| Diagnosis | 0.044 | |||
| Peptic ulcer | 182 (37.8) | 131 (41.6) | 51 (30.7) | |
| Adenoma | 210 (43.7) | 128 (40.6) | 82 (49.4) | |
| Adenocarcinoma | 42 (8.7) | 30 (9.5) | 12 (7.2) | |
| Others | 47 (9.8) | 26 (8.3) | 21 (12.7) | |
| PPI | 0.002 | |||
| Lansoprazole | 434 (90.2) | 296 (94) | 138 (83.1) | |
| Pantoprazole | 39 (8.1) | 16 (5.1) | 23 (13.9) | |
| Esomeprazole | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Rabeprazole | 6 (1.2) | 2 (0.6) | 4 (2.4) | |
| Alcohol | 110 (25.9) | 76 (27.9) | 34 (22.4) | 0.209 |
| Smoking | 110 (25.9) | 83 (30.5) | 27 (17.8) | 0.004 |
| BMI (kg/m2) | 24±3.2 | 23.7±3.4 | 24.5±2.7 | 0.021 |
Table 2
Eradication Rates for 7-day Tailored Treatment Regimens
| Analysis | Eradication rates for tailored treatment regimens | |||
|---|---|---|---|---|
| Overall | S groupa | R groupa | p-valueb | |
| 7-day STT | 7-day BQT | |||
| PP analysis | 89.4 (379/424) | 89.7 (244/272) | 88.8 (135/152) | 0.775 |
| ITT analysis | 78.8 (379/481) | 77.5 (244/315) | 81.3 (135/166) | 0.233 |
Table 3
Eradication Rates for 14-day Tailored Treatment Regimens




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download