Abstract
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory disease. IgG4-RD can affect any organ system, including the pancreas, bile ducts, salivary glands, mesentery, and retroperitoneum. On the other hand, small intestine involvement is extremely rare. This paper describes a case of IgG4-RD involving the small bowel, particularly at the distal ileum. An 81-year-old female was admitted to the authors’ hospital complaining of abdominal pain, dyspepsia, and hematochezia. The laboratory tests, including tumor markers and IgG4, were within normal limits. A colonoscopy did not show any abnormal findings. Abdominal computed tomography revealed segmental aneurysmal dilatation and wall thickening at the distal ileum, suggesting malignant conditions, such as small bowel lymphoma. The patient underwent an exploratory laparoscopy and ileocecectomy to differentiate a malignancy. A histopathology examination revealed dense lymphoplasmacytic infiltration, storiform fibrosis, and IgG4-positive plasma cells (>50 per high power field). The patient was finally diagnosed with IgG4-RD. The patient was followed up in the outpatient clinic for five years without recurrence. This paper suggests that a radical resection without maintenance therapy can be a treatment option, particularly when the IgG4-RD manifests as a localized gastrointestinal tract lesion.
Immunoglobulin G4-related disease (IgG4-RD) is a systemic immune-mediated fibroinflammatory disease. The disease can be characterized by the elevated level of IgG4 in the serum. On the other hand, a high IgG4 level is not diagnostic because it can be associated with many other conditions, such as allergic, infectious, and malignant diseases.1 Furthermore, it can present differently in many organs: the pancreas, bile ducts, gallbladder, thyroid, lungs, pericardium, salivary glands, kidneys, mesentery, periorbital tissues, prostate, skin, and retroperitoneum.1,2 Nevertheless, the small bowel involvement of IgG4-RD is rare. This paper presents a case of IgG4-RD involving the small bowel, particularly the distal ileum.
An 81-year-old female was admitted to the authors’ hospital complaining of intermittent abdominal pain and dyspepsia accompanied by hematochezia for one month. She did not have any systemic diseases except hypertension for 25 years. She denied any history of using alcohol and tobacco, or other recreational substances.
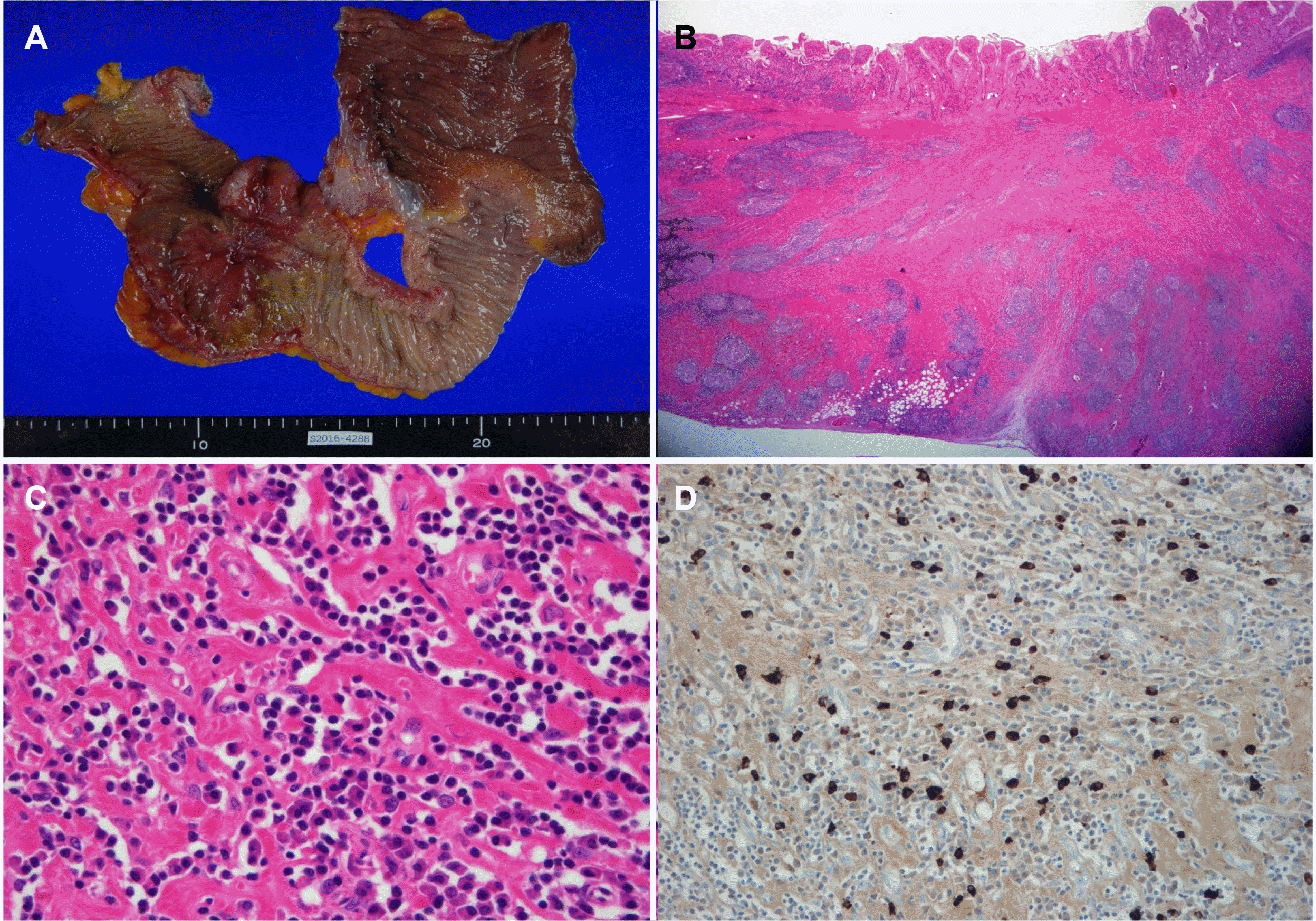
Upon admission, the blood pressure, pulse rate, and body temperature were 125/74 mmHg, 64/min, and 36.5℃, respectively. The laboratory data were as follows: white blood cell count 4,730/mm3, hemoglobin 9.8 g/dL, hematocrit 30.7%, platelet count 351,000/mm3, aspartate aminotransferase 17 IU/L, alanine aminotransferase 15 IU/L, alkaline phosphatase 84 IU/L, lactate dehydrogenase 203 U/L, amylase 38 U/L, C-reactive protein 0.3 mg/dL, Carcinoembryonic antigen 1.4 ng/mL, CA19-9 7.4 U/mL, IgG1 533.2 mg/dL, IgG2 166.1 mg/dL, IgG3 30.5 mg/dL, and IgG4 18.9 mg/dL. A colonoscopy did not show any abnormal findings in the terminal ileum (Fig. 1). Abdominal computed tomography revealed segmental aneurysmal dilatation and irregular wall thickening in the distal ileum (Fig. 2). Based on these clinical and radiology findings, malignant disease, such as small bowel lymphoma was suspected as a presumptive diagnosis. The patient underwent an exploratory laparoscopy. An approximately 4 cm mass encircling the distal ileum was observed in the surgical field. Therefore, an extensive resection and lymph node dissection were performed under the suspicion of a malignancy. The patient underwent a right hemicolectomy, which involved removing the cecum and ascending colon (9 cm in length), with a distal ileal resection (28 cm in length) and lymph node dissection. The surgical gross specimen showed a 4×4 cm ulceroinfiltrative lesion of the distal ileum (Fig. 3A). The ulceroinfiltrative lesion was located 7 cm and 11 cm apart from the ileal resection margin and ileocecal valve, respectively. The lymph nodes, the remaining ileal mucosa, and the colonic mucosa were intact. A histological examination revealed chronic ileitis with ulceration and transmural lymphoid aggregates (Fig. 3B), dense lymphoplasmacytic infiltration, and storiform fibrosis (Fig. 3C). Immunohistochemistry revealed many IgG4-positive plasma cells (>50 per high power field [HPF]) in the distal ileum, indicative of IgG4-RD (Fig. 3D). Therefore, the patient was finally diagnosed with IgG4-RD involving the distal ileum. The patient was discharged on postoperative day 10 without postoperative complications and was followed up via the outpatient clinic for five years without the recurrence of IgG4-RD.
Since IgG4-RD was recognized as a disease entity 20 years ago, it is now accepted that IgG4-RD could be manifested as many mimics of inflammatory or malignant diseases. This pleomorphic feature of IgG4-RD makes a differential diagnosis difficult, particularly when presenting as a mass-like lesion. Moreover, the diagnostic difficulty of IgG4-RD leads to the development of several guidelines and diagnostic criteria from several study groups.3-6 As IgG4-RD is a systemic autoimmune-related disease, the treatment strategies and disease prognosis differ according to the organs involved. Therefore, a new guideline focusing on the involvement of the digestive tract was published in 2020 by the European Society of Gastroenterology & Surgery groups.7 The European guideline highlights the need for a comprehensive work-up. The HISORt criteria is used as diagnostic criteria, named by the initials of the following words: “h”istopathological and “i”maging features, “s”erum IgG4 elevation, other “o”rgan involvement, and “r”esponse to steroid “t”herapy. Furthermore, a definite diagnosis of IgG4-RD is made when fulfilling the organ-specific diagnostic criteria given the established organ-specific diagnostic criteria.4,6
In laboratory tests, the serum IgG and IgG4 elevation, autoantibodies including antinuclear antibody, and rheumatoid factor can help diagnose IgG4-RD, even though such laboratory findings are not specific for the diagnosis. The histological examination revealed abundant IgG4+ plasma cells within the affected tissues as distinct features: proposed diagnostic criteria of >50 IgG4 + plasma cells per HPF and an IgG4 +/IgG + ratio > 40%.1,4,7 Other important pathological features include storiform fibrosis, lymphoplasmacytic infiltration, and phlebitis. In the present case, although the serum IgG4 was within the normal range, storiform fibrosis, dense lymphoplasmacytic infiltration, and IgG4+ plasma cells >50/HPF were demonstrated in the resected tissue, making a definite diagnosis of IgG4-RD involving the distal ileum of the small bowel. In addition, this case showed that long-term remission without relapse had been maintained after a surgical resection for approximately five years. Watanabe et al. reported that a surgical resection without steroid maintenance therapy could achieve the long-term remission of IgG4-RD.8 IgG4-RD has a high relapse rate of approximately 50% after the discontinuation of the steroid, even though steroid is a very effective agent for the treatment of IgG4-RD,9 and it can develop into malignant disease, particularly for IgG4-related pancreatitis (also known as autoimmune pancreatitis type 1).10-12 Therefore, a surgical resection for IgG4-RD can be a treatment option, especially when the IgG4-RD manifests as a localized lesion of the gastrointestinal tract with concern for possible malignancy as a differential diagnosis.
IgG4-RD can involve various organs, such as the pancreas, bile ducts, gallbladder, thyroid, liver, kidneys, and lungs. Although IgG4-RD can manifest only in a single organ, as in the present case, the involvement of multiple organs simultaneously is a distinct feature of IgG4-RD. Therefore, when IgG4-RD is suspected, it is important to determine if the other organs are involved.
The pancreas and bile ducts are the most frequently affected organs in IgG4-related pancreatitis and IgG4-related cholangitis. On the other hand, the other gastrointestinal tracts are rarely involved, even though a few reports of IgG4-RD involving the esophagus or stomach have been published. Furthermore, small bowel involvement has been scarcely reported.8,13-18
The symptoms of IgG4-RD can differ according to the involved organs. In particular, in hepatobiliary involvement (IgG4-related cholangitis or IgG4-related pancreatitis), IgG4-RD usually presents with obstructive jaundice, weight loss, and abdominal discomfort. On the other hand, the symptoms related to IgG4-RD involving the small bowel have not been well reported. Therefore, considering the previous reports of IgG4-RD of the small bowel, the symptoms may include recurrent abdominal pain with/without vomiting or bloating and obscure bleeding without definite lesions on routine examinations.
In elderly patients presenting with abdominal pain, dyspepsia, and hematochezia, it is crucial to differentiate these symptoms from infectious enterocolitis, ischemic colitis, and gastrointestinal tract malignancy. In the present case, there were no signs of infection, such as fever, and the symptoms were not acute, which helped to exclude infectious enterocolitis and ischemic colitis.
Regarding the radiology findings of IgG4-RD involving the small bowel, Ko et al.14 described the following radiology findings of a computed tomography scan: losses of mural stratification, irregularly thickened wall, and segmental aneurysmal dilatation, which are similar findings of our case.
In summary, IgG4-RD can mimic lymphoproliferative disorders, even in the distal ileum of the small bowel. Furthermore, to the best of our knowledge, this is the first case report demonstrating the long-term clinical outcomes after radical surgery for IgG4-RD solely involving the distal ileum of the small bowel. This case suggests that a radical resection without maintenance therapy can be a treatment option, particularly when the IgG4-RD manifests as a localized gastrointestinal tract lesion.
REFERENCES
1. Löhr JM, Vujasinovic M, Rosendahl J, Stone JH, Beuers U. 2022; IgG4-related diseases of the digestive tract. Nat Rev Gastroenterol Hepatol. 19:185–197. DOI: 10.1038/s41575-021-00529-y. PMID: 34750548.
2. Lee YS, Lee SH, Lee MG, et al. 2013; Immunoglobulin g4-related disease mimicking unresectable gallbladder cancer. Gut Liver. 7:616–620. DOI: 10.5009/gnl.2013.7.5.616. PMID: 24073322. PMCID: PMC3782679.
3. Moon SH, Kim MH. 2022; Autoimmune pancreatitis and immunoglobulin G4-related sclerosing cholangitis: Past, present, and future. Korean J Gastroenterol. 80:107–114. DOI: 10.4166/kjg.2022.102. PMID: 36156034.
4. Umehara H, Okazaki K, Kawa S, et al. 2021; The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol. 31:529–533. DOI: 10.1080/14397595.2020.1859710. PMID: 33274670.
5. Wallace ZS, Naden RP, Chari S, et al. 2020; The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-related disease. Arthritis Rheumatol. 72:7–19. DOI: 10.1002/art.41120. PMID: 31793250.
6. Umehara H, Okazaki K, Nakamura T, et al. 2017; Current approach to the diagnosis of IgG4-related disease - Combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol. 27:381–391. DOI: 10.1080/14397595.2017.1290911. PMID: 28165852.
7. Löhr JM, Beuers U, Vujasinovic M, et al. 2020; European Guideline on IgG4-related digestive disease - UEG and SGF evidence-based recommendations. United European Gastroenterol J. 8:637–666. DOI: 10.1177/2050640620934911. PMID: 32552502. PMCID: PMC7437085.
8. Watanabe A, Goto T, Kamo H, et al. 2018; Resection of lesions in the ileum of patients with IgG4-related disease may ameliorate disease progression without steroid administration. Surg Case Rep. 4:148. DOI: 10.1186/s40792-018-0546-9. PMID: 30594958. PMCID: PMC6311175.
9. Lee HW, Moon SH, Kim MH, et al. 2018; Relapse rate and predictors of relapse in a large single center cohort of type 1 autoimmune pancreatitis: long-term follow-up results after steroid therapy with short-duration maintenance treatment. J Gastroenterol. 53:967–977. DOI: 10.1007/s00535-018-1434-6. PMID: 29362937.
10. Shiokawa M, Kodama Y, Yoshimura K, et al. 2013; Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol. 108:610–617. DOI: 10.1038/ajg.2012.465. PMID: 23318486.
11. Hart PA, Law RJ, Dierkhising RA, Smyrk TC, Takahashi N, Chari ST. 2014; Risk of cancer in autoimmune pancreatitis: a case-control study and review of the literature. Pancreas. 43:417–421. DOI: 10.1097/MPA.0000000000000053. PMID: 24622072.
12. Lee KW, Chang JH, Kim J, et al. 2020; Metachronous development of peritoneal carcinomatosis in a patient with autoimmune pancreatitis. Korean J Gastroenterol. 75:356–361. DOI: 10.4166/kjg.2020.75.6.356. PMID: 32581208.
13. Wong DD, Pillai SR, Kumarasinghe MP, et al. 2012; IgG4-related sclerosing disease of the small bowel presenting as necrotizing mesenteric arteritis and a solitary jejunal ulcer. Am J Surg Pathol. 36:929–934. DOI: 10.1097/PAS.0b013e3182495c96. PMID: 22367294.
14. Ko Y, Woo JY, Kim JW, et al. 2013; An immunoglobulin G4-related sclerosing disease of the small bowel: CT and small bowel series findings. Korean J Radiol. 14:776–780. DOI: 10.3348/kjr.2013.14.5.776. PMID: 24043971. PMCID: PMC3772257.
15. Ciccone F, Ciccone A, Di Ruscio M, et al. 2018; IgG4-related disease mimicking Crohn's disease: A case report and review of literature. Dig Dis Sci. 63:1072–1086. DOI: 10.1007/s10620-018-4950-6. PMID: 29417330.
16. Campos-Murguía A, Martinez-Garcia CL, Chable-Montero F, Zamora-Nava LE. 2023; Multifocal ulcerating stenosing enteritis as a novel manifestation of immunoglobulin G4-related disease. Endoscopy. 55(S 01):E163–E164. DOI: 10.1055/a-1952-0490. PMID: 36307068. PMCID: PMC9829813.
17. Fujita K, Naganuma M, Saito E, et al. 2012; Histologically confirmed IgG4-related small intestinal lesions diagnosed via double balloon enteroscopy. Dig Dis Sci. 57:3303–3306. DOI: 10.1007/s10620-012-2267-4. PMID: 22695887.
18. Hiyoshi Y, Oki E, Zaitsu Y, et al. 2014; IgG4-related disease of the ileocecal region mimicking malignancy: A case report. Int J Surg Case Rep. 5:669–672. DOI: 10.1016/j.ijscr.2014.08.003. PMID: 25194601. PMCID: PMC4189076.
Fig. 2
Abdominal computed tomography shows segmental aneurysmal dilatation and wall thickening at the distal ileum (arrow) on axial (A) and coronal view (B).
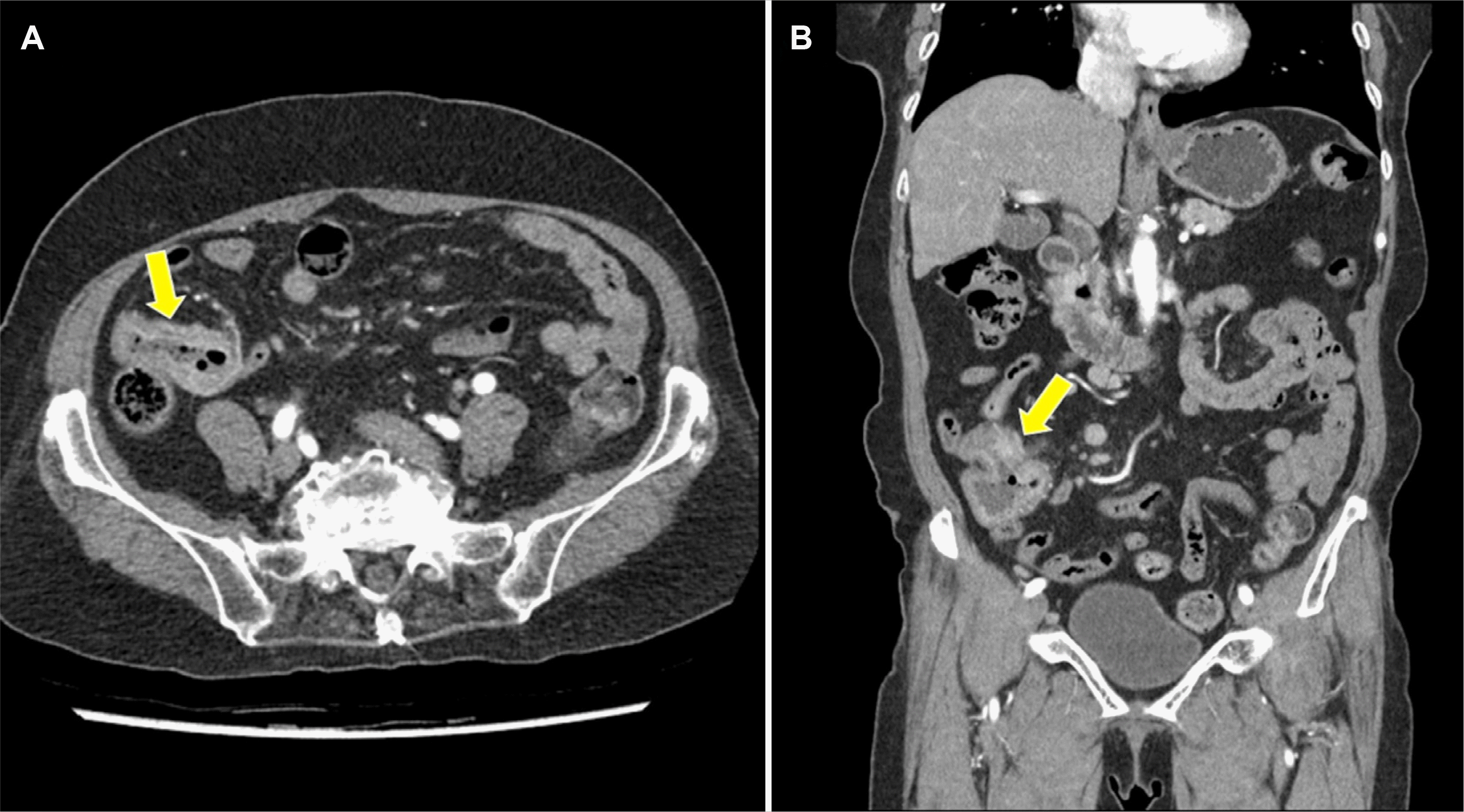
Fig. 3
(A) Gross specimen from segmental bowel resection shows a huge ulceroinfiltrative lesion at the distal ileum. (B) Pathologic findings with a low magnification view reveal transmural inflammation with many lymphoid follicles and sclerotic fibrosis (H&E, ×10). (C) Inflammatory infiltrates predominantly comprise plasma cells, lymphocytes, and some eosinophils (H&E, ×200). (D) Immunohistochemical stain reveals many IgG4-positive cells with more than 50 cells per high power field (×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download