Abstract
The results of the IMbrave150 study have led to widespread use of the combination therapy of atezolizumab and bevacizumab as a first-line treatment for unresectable or metastatic hepatocellular carcinoma (HCC). Compared to traditional cytotoxic chemotherapy agents, immune checkpoint inhibitors show a spectrum of side effects ranging from mild side effects such as skin rash to potentially severe systemic effects such as myocarditis. We present a case of transverse myelitis diagnosed during the treatment of HCC with atezolizumab and bevacizumab combination therapy.
Hepatocellular carcinoma (HCC) is the fourth-most common cause of cancer-related death worldwide, and treatment options for this condition are determined based on tumor stage and patient performance.1,2 Recently, the IMbrave150 study ―a phase 3 clinical trial―evaluated the efficacy and safety of atezolizumab in combination with bevacizumab versus sorafenib as a first-line treatment for unresectable or metastatic HCC.3 The success of that study, along with the emergence of other drug options, has led to the inclusion of the combination of atezolizumab and bevacizumab as a representative treatment for unresectable HCC in the guidelines.4-6
Immune checkpoint inhibitors (ICI) such as atezolizumab can result in immune-related adverse events as a result of excessive reaction by the immune system, with common side effects affecting the gastrointestinal tract, endocrine system, skin, and liver, and less frequently, the nervous, cardiovascular, respiratory, musculoskeletal, hematologic, and renal systems.7 Most side effects are mild, but very serious and even fatal nervous system or cardiac events have been reported in rare cases.8,9 Therefore, it is important to be cautious about immune-related adverse events that can lead to serious complications, even if they are rare.
This case report presents a rare occurrence of transverse myelitis in a patient with hepatocellular carcinoma following treatment with the combination of atezolizumab and bevacizumab, and it highlights the diagnosis and treatment of a neurologic adverse event associated with ICIs.
A 55-year-old male who had been undergoing regular surveillance for liver cirrhosis was diagnosed with multiple hepatocellular carcinoma and portal vein thrombosis, for which he decided to undergo systemic therapy (Fig. 1). He had a past history of chronic hepatitis C infection 3 years prior, which was successfully treated with direct-acting antiviral therapy, thereby achieving sustained virologic response 12. He also had type 2 diabetes mellitus (DM). At the time of diagnosis of HCC, his liver function was normal, and there were no other complications except for grade 1 esophageal varices as a complication of liver cirrhosis. His DM was also well controlled with oral medication, so we decided to administer atezolizumab and bevacizumab combination therapy. During the four cycles of atezolizumab and bevacizumab combination therapy that were administered every three weeks, there were no significant complications, and the treatment response was evaluated as stable disease based on liver dynamic CT.
After the fourth cycle of chemotherapy, 2 weeks later he fell down at home, causing knee pain, but he received no associated treatment. 3 weeks after chemotherapy, He was later admitted to the hospital through the emergency room due to loss of consciousness. He was diagnosed with septic knee and sepsis caused by Staphylococcus aureus, for which he was treated with IV antibiotics. During treatment, he developed mild ascites, which was managed with diuretics (oral furosemide 20 mg/day and oral spironolactone 50 mg/day). He also experienced a temporary decrease in liver function, with a decrease in serum albumin levels (baseline: 3.5 g/dL, event: 2.4 g/dL) and an increase in total bilirubin levels (baseline: 1.5 mg/dL, event: 6.1 mg/dL). We discharged the patient about 7 weeks after chemotherapy for more intensive rehabilitation treatment due to his knee pain and walking difficulties, despite his improved overall condition after sufficient treatment. We confirmed his condition in an outpatient setting and decided to continue systemic therapy. A few days later, it was 3 months after the fourth chemotherapy, during an outpatient visit, he was found to have decreased muscle strength in his left upper limb, and he was readmitted after a consultation with the neurology department.
At the time of readmission, neurological examination showed a deterioration in muscle strength to grade 3 in both lower limbs and the left upper limb, but there were no abnormal sensory levels or urinary or fecal incontinence. Brain MRI was examined, and stroke was excluded with normal findings. Laboratory tests showed albumin 3.2 g/dL, Prothrombin time international normalized ration 1.22 total bilirubin 1.8 mg/dL (normal range 0.2–1.2), Creatinine kinase 44 U/L (normal range 0–171), Lactate dehydrogenase 820 U/L (normal range 140–480), Aspartate transaminase/Alanine transaminase 112/121 U/L (normal range 0–40, 0–40), normal thyroid function, negative antinuclear antibodies, negative HIV antigen/ antibody test, and negative syphilis test. Anti-myelin oligodendrocyte glycoprotein Ab and anti-aquaporin 4 Ab, which are specific biomarkers of neuromyelitis optica, were not detected. Cerebrospinal fluid examination showed normal opening pressure without pleocytosis, and oligoclonal band IgG was type 1 negative. To evaluate for metastasis of HCC as well as differentiate from other conditions, brain and spine MRIs were performed. Diffuse spinal cord signal changes with contrast enhancement were observed on the whole T-spine MRI, which was compatible with long extensive transverse myelitis (Fig. 2). First-line treatment for transverse myelitis is high-dose glucocorticoids, which can cause serious side effects in patients with liver cirrhosis and DM. Therefore, we opted for five sessions of plasmapheresis as an alternative therapy, which was completed without any significant side effects. During a follow-up outpatient visit 2 weeks later, grade 4 muscle strength recovery was observed, and the patient is currently undergoing rehabilitation treatment as of this writing.
According to data from the Korean Nationwide Cancer Registry, advanced HCC with Barcelona Clinic Liver Cancer stage C accounts for 39.0% of first-time liver cancer diagnoses.10 Until about three years ago, Sorafenib was the oldest first-line treatment for advanced HCC, and was exclusively used for nearly 10 years. Sorafenib has demonstrated modest effectiveness, having been shown to achieve a survival benefit of less than 3 months, a tumor response rate below 5%, and a median survival time of under 1 year.11 Although there had been various attempts made using different treatment methods, none had shown better efficacy than sorafenib for years. However, in the IMbrave 150 study, atezolizumab-bevacizumab demonstrated significant improvements in overall survival and progression-free survival compared to sorafenib.3 The atezolizumab-bevacizumab group showed reduced early discontinuation, decreased side effects, and a significantly higher tumor response rate compared to the sorafenib group.3 As a result, the combination therapy of atezolizumab-bevacizumab has become the first option in systemic therapy for advanced HCC in the current treatment guidelines.4-6
While ICIs are known for their excellent cancer treatment performance and minimal side effects, they induce different side effects from traditional cytotoxic chemotherapies, which is possibly attributable to their impact on the body's immune system, although the precise pathophysiology is not fully understood12 Immune-related adverse events can occur in various organs with varying frequencies and severity, including skin lesions such as rash and pruritus, as well as colitis, pneumonitis, hypophysitis, thyroid dysfunction, hepatitis leading to severe liver failure, and myocarditis.7 At present, there is no prospective research on how to manage and treat immune-related adverse events, so treatment for these symptoms is based on clinical experience and may involve ICI discontinuation or steroid therapy depending on the severity of the symptoms and the patient's condition.7
The diagnosis of transverse myelitis requires comprehensive evaluation including clinical presentation, neuroimaging, and spinal cord inflammation. MRI is essential for confirming the presence of compressive lesions in the spine and brain, while cerebrospinal fluid analysis and immunohistochemical tests are also needed.13 Moreover, differential diagnoses such as neuromyelitis optica and multiple sclerosis should be ruled out.14 Differential diagnosis from transverse myelitis is possible based on the characteristic imaging findings and cerebrospinal fluid findings of multiple sclerosis. Brain MRI shows periventricular and juxtacortical lesions. In particular, in contrast enhancement, elliptical or ring-shaped lesions may be seen. Spinal cord MRI usually shows spinal cord lesions that involve less than 2 vertebrae. In cerebrospinal fluid, oligoclonal bands are observed in more than 90% of patients with multiple sclerosis, and the immunoglobulin G index is increased in more than 60%.15 This patient underwent brain and spinal MRI and cerebrospinal fluid tests to differentiate between multiple sclerosis and optic neuritis through consultation with a neurologist, and special biochemical tests such as anti-aquaoprin 4 Ab were performed. High-dose glucocorticoids are the first-line treatment for acute transverse myelitis, and plasma exchange may be considered if initial therapy is ineffective.13
There are cases of neuromyelitis optica and transeversemyelitis after ICI use. The case reported by Moodie et al.16 was a patient with transverse myelitis after using durvalumab for non small cell lung cancer. He was treated with steroids, but died during the tapering process due to a decline in his condition. Another case was neuromyelitis optica after nivolumab in lung squamous cell carcinoma reported by Narumi et al.17 He was treated with steroids, but he didn't respond well, so tried plasma exchange, which also didn't work. The third report is a case of transverse myelitis following atezolizumba use in a patient with metastatic small cell lung cancer reported by Esechie et al.18 After 5 days of steroid treatment, he received 3 days of plasma exchange therapy, but the outcome was not favorable.
In summary, we report a case of transverse myelitis during atezolizumab-bevacizumab treatment for advanced HCC that highlights the potential for unexpected side effects with new first-line therapies. This rare but serious condition can result in significant disability, thus emphasizing the importance of prompt diagnosis and management through collaboration among healthcare providers from various departments.
REFERENCES
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. 2019; A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 16:589–604. DOI: 10.1038/s41575-019-0186-y. PMID: 31439937. PMCID: PMC6813818.
2. Llovet JM, Kelley RK, Villanueva A, et al. 2021; Hepatocellular carcinoma. Nat Rev Dis Primers. 7:6. DOI: 10.1038/s41572-020-00240-3. PMID: 33479224.
3. Finn RS, Qin S, Ikeda M, et al. 2020; Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 382:1894–1905. DOI: 10.1056/NEJMoa1915745. PMID: 32402160.
4. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022; 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 28:583–705. DOI: 10.3350/cmh.2022.0294. PMID: 36263666. PMCID: PMC9597235.
5. Su GL, Altayar O, O'Shea R, et al. 2022; AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 162:920–934. DOI: 10.1053/j.gastro.2021.12.276. PMID: 35210014.
6. Reig M, Forner A, Rimola J, et al. 2022; BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76:681–693. DOI: 10.1016/j.jhep.2021.11.018. PMID: 34801630. PMCID: PMC8866082.
7. Martins F, Sofiya L, Sykiotis GP, et al. 2019; Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 16:563–580. DOI: 10.1038/s41571-019-0218-0. PMID: 31092901.
8. Li C, Bhatti SA, Ying J. 2022; Immune checkpoint inhibitors-associated cardiotoxicity. Cancers (Basel). 14:1145. DOI: 10.3390/cancers14051145. PMID: 35267453. PMCID: PMC8909315.
9. Cuzzubbo S, Javeri F, Tissier M, et al. 2017; Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer. 73:1–8. DOI: 10.1016/j.ejca.2016.12.001. PMID: 28064139.
10. Yoon JS, Lee HA, Kim HY, et al. 2021; Hepatocellular carcinoma in Korea: An analysis of the 2015 Korean nationwide cancer registry. J Liver Cancer. 21:58–68. Retracted and republished in : J Liver Cancer 2022;22:207. DOI: 10.17998/jlc.21.1.58. PMID: 37384267. PMCID: PMC10035724.
11. Cheng AL, Kang YK, Chen Z, et al. 2009; Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34. DOI: 10.1016/S1470-2045(08)70285-7. PMID: 19095497.
12. Postow MA, Sidlow R, Hellmann MD. 2018; Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 378:158–168. DOI: 10.1056/NEJMra1703481. PMID: 29320654.
13. Beh SC, Greenberg BM, Frohman T, Frohman EM. 2013; Transverse myelitis. Neurol Clin. 31:79–138. DOI: 10.1016/j.ncl.2012.09.008. PMID: 23186897. PMCID: PMC7132741.
14. Kitley JL, Leite MI, George JS, Palace JA. 2012; The differential diagnosis of longitudinally extensive transverse myelitis. Mult Scler. 18:271–285. DOI: 10.1177/1352458511406165. PMID: 21669935.
15. Kim NH. 2016; Differential diagnosis between multiple sclerosis and neuromyelitis optica spectrum disorder. J Korean Neurol Assoc. 34:290–296. DOI: 10.17340/jkna.2016.4.2.
16. Moodie T, Alshaqi O, Alchaki A. 2022; Longitudinal extensive transverse myelitis after chemoradiation therapy with durvalumab, a rare complication: case report. BMC Neurol. 22:107. DOI: 10.1186/s12883-022-02576-7. PMID: 35305566. PMCID: PMC8933995.
17. Narumi Y, Yoshida R, Minami Y, et al. 2018; Neuromyelitis optica spectrum disorder secondary to treatment with anti-PD-1 antibody nivolumab: the first report. BMC Cancer. 18:95. DOI: 10.1186/s12885-018-3997-2. PMID: 29361915. PMCID: PMC5781276.
18. Esechie A, Fang X, Banerjee P, Rai P, Thottempudi N. A case report of longitudinal extensive transverse myelitis: immunotherapy related adverse effect vs. COVID-19 related immunization complications. Int J Neurosci. 2022; Apr. 3. doi: 10.1080/00207454.2022.2050907. DOI: 10.1080/00207454.2022.2050907. PMID: 35369847.
Fig. 1
The patient's initial liver dynamic computer tomography shows multiple nodules in both hepatic lobes that demonstrate enhancement in the arterial phase (A, B), and washout in portal phase (C). Tumor thrombosis of the right portal vein is observed (D).
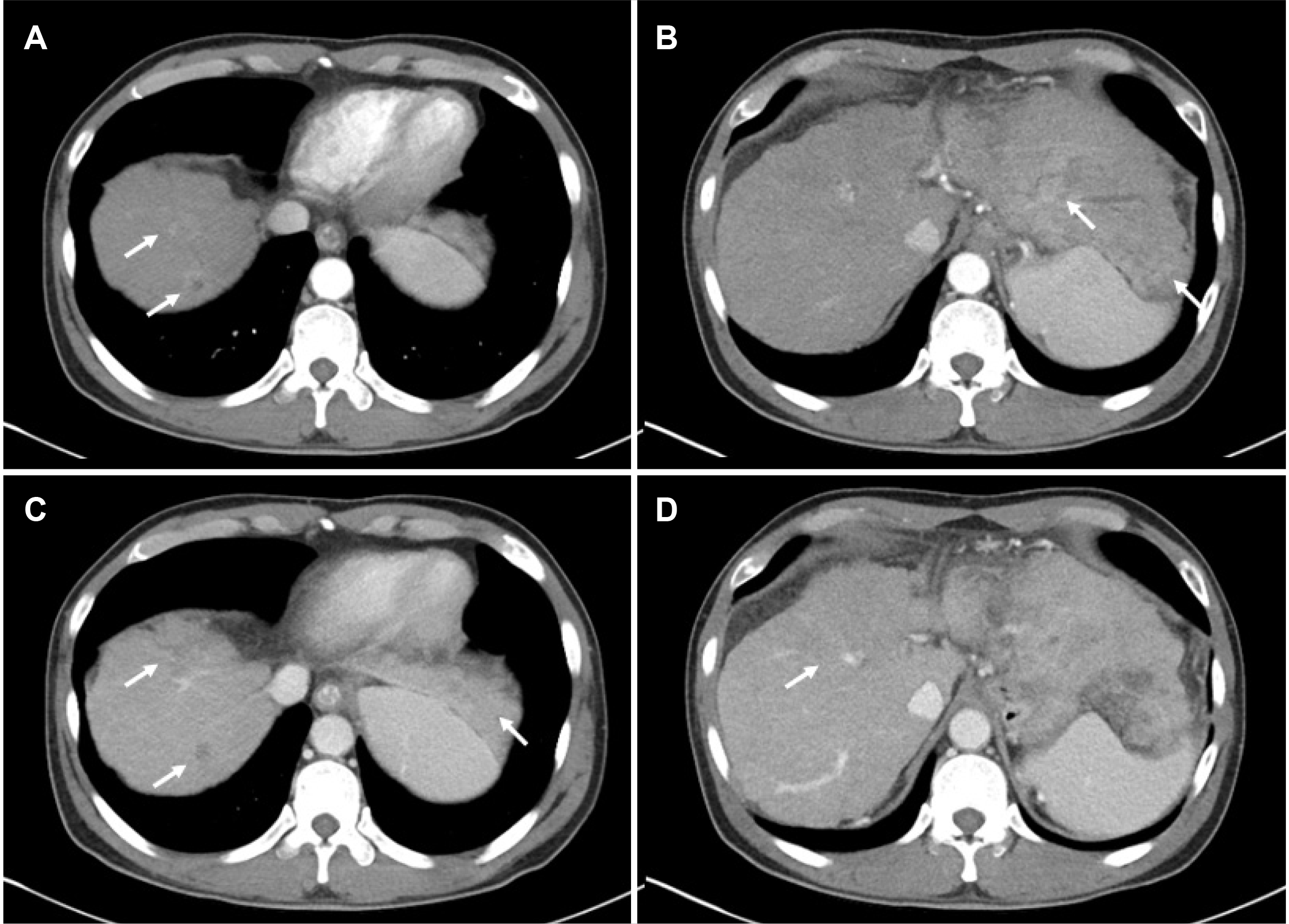
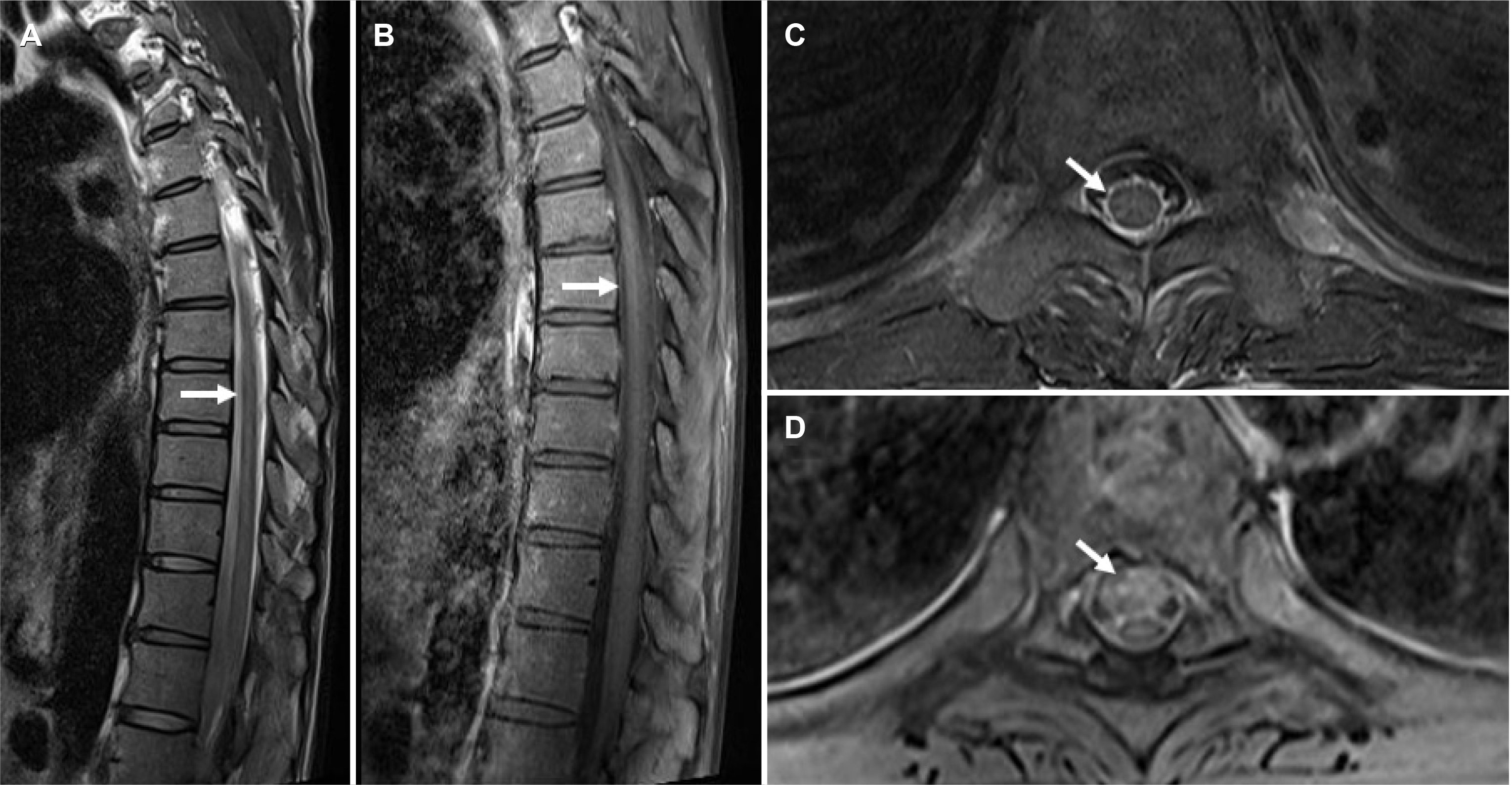
Fig. 2
Thoracic MRI findings of the patient. Diffuse spinal cord high signal intensity on T2 weighted sagittal image was observed and there was abnormal contrast enhancement on T1 sagittal image (A, B). On axial image, subtle high signal intensity was observed on T8 level with prominent T1 contrast enhancement (C, D).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download