Abstract
Background
In Vietnam, the rate of absolute uterine factor infertility is increasing, but no study has been published on uterine transplantation. The present study was designed to comprehensively observe the canine uterine anatomy and to examine the possibility of using a living canine donor for uterine transplantation training and further research.
Methods
Ten female Vietnamese mixed-breed dogs were sacrificed for anatomical research, and 15 additional pairs were used to evaluate the novel uterine transplant model.
Results
The anatomic features of the canine uterus differed considerably from those of the human uterus, with the uterine vessels originating from branches of the pudendal vessels (also known as the vaginal vessels). The uterine vascular pedicle had a small diameter (1 to 1.5 mm for arteries and 1.2 to 2.0 mm for veins) and required manipulation under a microscope. To perform uterine transplantation, the donor specimen’s artery and vein lengths were successfully reconstructed by anastomosis between both sides of the vasculature using autologous Y-shaped subcutaneous veins. The living-donor uterine transplantation model constructed in this study was feasible, with the transplanted uterus surviving in 86.7% of cases (13/15).
Infertility affects between 10% and 15% of couples of reproductive age. Within that population, approximately one in 500 women has absolute uterine infertility [1]. These women either lack a uterus from birth, lose the uterus through hysterectomy, or have a uterine defect impacting implantation or pregnancy. Uterine transplantation appears to be a feasible option for women without a uterus to bear genetic children. The first childbirth following uterine transplantation from a living donor occurred in Gothenburg, Sweden, in September 2013 [2].
Training on animal models is a prerequisite to implementing a human uterine transplantation program. Numerous animal models have been developed involving mice, rats, rabbits, dogs, cats, sows, and sheep [3]. The first mention of uterine transplantation, in 1927, involved a dog model but included scant reporting of the methodology and results. Experimental work on uterine transplantation was reinitiated in the 1960s and the 1970s and primarily involved autotransplantation. In that research, the vascular anastomosis was examined and compared to achieve immediate reperfusion and attachment of the uterus to an abdominal surface. The goal of this process was to produce gradual revascularization through the outgrowth of new blood vessels. At the level of the internal iliac vessels, vascular anastomosis was first used in en bloc autotransplantations of the canine uterus, oviducts, and ovaries. In 1964, Zhordania and Gotsiridze [4] reported successful replantation of the uterus and its appendages in rabbits, dogs, and particularly sheep. They used omentopexy for revascularization, yielding successful pregnancy in some instances. In 1966, Eraslan et al. [5] were the first to perform autotransplantation of the uterus with its adnexal organ by vascular anastomosis in canines. As uterine grafts involve iliac vessel intervention, all canine models of uterine transplantation established with reapproximation of vessels included deceased donors [5-8].
In Vietnam, no official report has been published on uterine transplantation. Given recent advances in the field, uterine transplants may soon be performed as a cure for absolute uterine infertility. Properly designed research on uterine transplantation in animal models will benefit the development of surgical methodologies applied to donors and recipients. Additional information on immunosuppressive medications and their effects on the mother and fetus would also be useful for human trials. In this study, we described the uterine anatomy of the dog and investigated a new canine living donor for uterine transplantation.
We conducted this study in compliance with the principles of the Declaration of Helsinki. Animals were treated in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication No. 86–23, revised 1985) and the Institutional Guide for the Care and Use of Laboratory Animals. All experimental protocols involving animals were approved by the Animal Care and Use Committee of Vietnam Military Medical University (Institutional Animal Care and Use Committee No. 14).
This prospective descriptive study was performed with adult female mixed-breed Vietnamese dogs. Ten female dogs were used to study the anatomic characteristics of the uterus, and 15 pairs of female dogs were used to verify the feasibility of the transplant model. Each recipient dog was assigned a number from N1 to N15. The study was executed at the Department of Practical and Experimental Surgery of Vietnam Military Medical University between February and May 2020.
The dogs were acclimated to the holding facility for at least 5 days before experimental manipulation. On the day of the experiment, the animals were brought to the operating theater individually. Two animal caretakers oversaw the administration of anesthesia. Each dog was fixed on the surgical table and anesthetized with ketamine (1,000 mg/10 mL) at a dose of 10 mg/kg. Anesthesia was performed by femoral injection at 0.5 mg/kg/min. With the dog supine, respiration was maintained via an endotracheal positive pressure respirator. At the end of the experiment, the animal was sacrificed using a high dose of intravenously injected ketamine.
For the first 2 days, CellCept (500 mg, Genentech) was pumped through a nasogastric tube at a dose of 1 g/day, and Solu-Medrol (40 mg, Pfizer Inc.) in 500 mL of 5% dextrose solution was injected into the femoral vein. This intravenous infusion of 40 mg of Solu-Medrol was administered twice daily, in the morning and afternoon. In the following days, only 40 mg of Solu-Medrol was used, in the form of an intramuscular (buttock) injection of two ampoules per day.
Antibiotics were used according to a prophylactic antibiotic regimen. A single dose of cefuroxime (750 mg) was injected intravenously 30 minutes prior to skin incision.
Before transplantation, the animals were injected intravenously with heparin (5,000 IU/5 mL) at a single dose of 300 IU/kg. After transplantation, defibrotide (80 mg/mL) was administered at a dose of 6.25 mg/kg body weight. Lovenox (40 mg/0.4 mL, Sanofi) was injected intravenously on the first day only, with one ampoule each (from a box of two ampoules) used in the morning and afternoon.
After analgesia, exsanguination was performed in the anatomical group through femoral catheterization. All blood was removed from the vascular system via flushing with physiological saline buffer-mixed heparin through the external jugular vein cannula. The vascular structure of the uterus was carefully dissected under magnification with surgical loupes (SOM2000E, Shanghai Link Instruments Co.).
A midline lower abdominal incision was used for both donor and recipient dogs. The peritoneum between the sigmoid colon and the common origin of the hypogastric arteries from the aortic bifurcation was incised to expose the donor specimen. This specimen was first prepared by carefully dissecting the main trunk of the common internal iliac artery and tracing it down to the uterine arteries. The uterine veins were exposed near the cervix and traced to the internal iliac vein and the junction of the common iliac vein. Distally, the uterus was divided across the vagina, and superiorly, the uterine cornua were transected, thus excluding the ovaries, since ovarian arterial anastomosis was deemed impractical due to the small vessel size. The uterine artery and vein were transected after the branch supplying the bladder. The specimen was quickly flushed with chilled histidine-tryptophan-ketoglutarate preservation solution (Custodiol 2,000 mL, Dr. Franz Köhler Chemie GmbH) containing heparin and transplanted into the recipient’s pelvis.
The recipient team performed surgery simultaneously with the donor team to reduce the ischemic period of the graft. The recipient procedure first involved hysterectomy. This procedure required the preservation of the uterine vascular pedicle for as long as possible and the maintenance of the vagina long enough to reapproximate with the donor vagina. Two stay sutures were placed on both sides of the vaginal stump to prevent it from contracting caudally. The uterine vascular stumps were trimmed and clamped with microvascular bulldog clamps. After vascular and vaginal preparation, the uterine graft was implanted. First, the graft vagina was sutured to the donor vaginal stump with two stay sutures in an interrupted fashion with an absorbable suture. Then, the graft’s vascular components were reconnected with those of the recipient with 9-0 nonabsorbable sutures in an end-to-end fashion under a ×10 microscopic lens (TAKAGI OM-9, Takagi Seiko Co.). Reperfusion was initiated immediately after the vascular anastomosis, followed by hemostasis supplementation. The graft was kept hypothermic during implantation by wrapping it in an ice-filled towel. The transplanted uterus was monitored for 3 hours after transplantation. During the first 24 hours, we focused on monitoring the whole body and vital functions of the animal. We used Doppler ultrasound to detect uterine perfusion.
X-ray imaging was performed on day 5 after uterine transplantation for a visual radiological examination of the transplanted uterus. After the animal was anesthetized, a catheter was inserted into the vagina and cervix under radiological guidance. The contrast medium Visipaque 320 (GE HealthCare) was injected through the catheter at doses of 4 and 6.5 mL. At 15 minutes after injection, a dorsoventral view was shown on an X-DR Static BT & WS device (EXAMION GmbH).
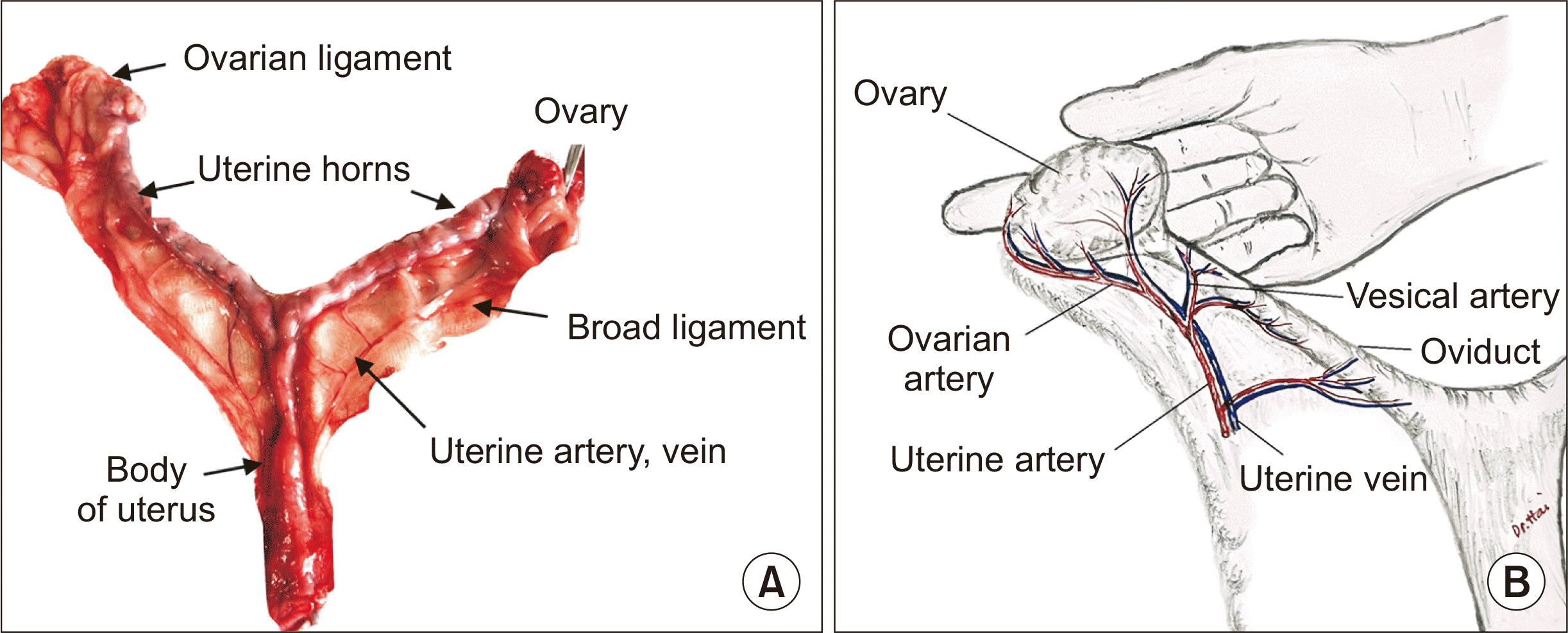
The ovaries of the dogs were located near each kidney. The ovary was enveloped by a “catcher’s mitt” tissue termed the infundibulum. This basket or mitt “catches” the eggs and transports them into the fallopian tubes. The fallopian tubes led to the uterus, where the fertilized egg is implanted and grows. The uterus had paired ovaries, fallopian tubes, paired uterine horns, and a short cervix. The uterine horns united to form the body of the uterus, which was joined to the vagina (Fig. 1A). The two horns were symmetrical and laterally oblate. In the present study, the average weight of the uterus (including the body, cervix, and two horns) was 23.3 g (range, 18–26 g). The average length of the uterine horn was 6.3±1.2 cm.
The vascular distribution of the vaginal artery was consistent across the 10 dogs. Two arteries on both sides supplied blood to the uterine body and horn. The uterine artery was the cranial branch of the vaginal arteries, which the internal pudendal artery gave rise to at the level of the sacroiliac joint. The vaginal artery formed an angle of approximately 45.0°–51.0° with the internal pudendal artery, with an average angle of 48.7°±3.2° (Table 1). This angle would make vessel dissection and anastomosis during graft implantation more challenging for novice surgeons. The uterine artery supplied a caudal vesical artery to the bladder and had ureteral and urethral branches. The uterine artery coursed cranially along the body and horn of the uterus in the broad ligament and anastomosed with the uterine branch of the ovarian artery in the mesometrium (Fig. 1B).
The diameters of the uterine artery and vein were 1–2 mm. The distance from the vesical artery bifurcation to the site where the uterine vessel ran close to the body of the uterus was 1.3 cm on average (range, 1.0–1.5 cm) (Table 1). The uterine artery split into an artery branch that fed the ovary, which ran along the fallopian tube, and an artery branch that fed the vagina.
The organ harvesting procedure was carried out with a microscope to minimize damage to the fragile canine small uterine vessels. All donor uteri (15/15) were successfully operated on and kept intact for the implantation (Fig. 2A). The mean organ procurement time was 75.6 minutes (range, 60–86 minutes) (Fig. 2B).
Since the vascular pedicle was collected from the vesicular vessel bifurcation, the donor specimen’s artery and vein lengths were relatively short and required reconstruction. This plastic step was done with four segments of autologous subcutaneous veins anastomosed to both sides of the uterine artery and vein with a 9-0 interrupted suture (Fig. 3A and B).
Regarding the length of the graft pedicle after angioplasty, the longest pedicle was 2.1 cm, while the shortest was 1.2 cm. The average length was 1.4±0.2 cm (Fig. 3C). In the early cases (n=3), due to concerns about high tension on the vascular anastomosis site, we tended to leave the pedicle at least 1.8 cm long. However, in later cases, we recognized that 1.3 to 1.5 cm of pedicle length was enough to prevent excessive tension on the anastomosis.
The first warm ischemia period extended from the time of vascular clamping until cold flushing. The second warm ischemic period occurred after the cold ischemia, when the vascular anastomoses were established. The average duration of the first warm ischemia period was 3.54±1.09 minutes, with a maximum of 6.3 minutes. Warm ischemia was time-consuming because of the small vessel size. After harvest, two uterine arteries on both sides were cannulated with a 23G needle for flushing. In earlier cases (n=5), the cannulation took longer than in later cases due to unfamiliarity with the microscope magnification. This time improved in the following cases, with one case requiring only 1 minute for cannulation (Fig. 4).
Regarding cold ischemia, the greatest time expenditure for ice preservation (65 minutes) occurred in the first case due to improper cooperation between the retrieval and implantation teams. The coordination between teams later improved, leading to a decrease in cool ischemia time; this duration most commonly fell between 41 and 50 minutes, accounting for 40% (6/15) of the samples (Fig. 4).
The maximum time for the recipient operation was 140 minutes, and the minimum was 85 minutes. The average time was 102.7±15.2 minutes (Fig. 4). This included time for angioplasty, in which the length of uterine vessels was augmented on both sides with subcutaneous veins.
In most cases (13 of 15, 86.67%), the uterine macromorphology after reperfusion was bright red and firm. In one case (N2), it was light pink and soft (6.67%), while in another (N1), it was congested and chalky (6.67%). Assessment under a microscope revealed Y-graft anastomoses with a clear pulse in 13 cases (86.67%). N2 exhibited low flow through arterial anastomoses (one case, 6.67%), and N1 had obstructed venous anastomoses (one case, 6.67%). Doppler ultrasound during the first 24 hours after transplantation was positive.
X-ray imaging on day 5 after uterine transplantation showed that the contrast agent was a little absorbed in the uterus and fallopian tubes. Interestingly, no contrast agent appeared in the abdomen (Fig. 5B).
The survival rate at 3 hours after transplantation was 100% (15/15). However, two dogs (N1 and N2) had obstructed venous anastomoses, which were the main complications observed in this study. The obstructed venous anastomoses led to the histological disorder of the transplanted uteri. No sign of urinary tract injury was observed in any canine after transplantation.
Animal models have been used to study uterine transplantation and develop techniques for successful human transplantation. Undeniably, animal models have played an essential role in the establishment of uterine transplantation, which is a highly complex and risky procedure [9]. While respecting the 3R requirements for humane animal research (replacement, refinement, and reduction), surgical training using large animal models, such as ewes, remains irreplaceable for teams wishing to initiate a uterine transplantation program [10]. However, ewes are uncommon in Vietnam, and the country lacks suitable raising conditions for research. Moreover, obtaining ewes for research is rather expensive. Other animal models that have been used for uterine transplantation research include mice [11], rats [12], rabbits [13], and pigs [14]. Among them, mice and rats are often used due to their small size, low cost, and ease of handling. However, their size limits the applicability of the results, as the size and structure of their uteri differ considerably from those of humans [15]. Pigs have been used more recently due to their larger size and more similar anatomy to humans, but they are expensive and require specialized care [16]. Hence, in this study, a canine model was chosen due to that animal’s prevalence, ease of care and handling, and reasonable cost.
The uterine anatomy of the Vietnamese mixed-breed dog used in this study has not been previously reported. Here, we show that the canine uterine artery differs entirely from the human uterine anatomy—in which the uterine artery extends directly from the internal iliac artery—with a smaller diameter of the vascular pedicle than that in humans. Choosing a canine model for research and technical training is challenging because of the large difference in overall size compared to humans, leading to corresponding differences in the vascular system. The critical point gained from this canine model was the reconstruction of the donor specimen’s artery and vein lengths via the anastomosis of both sides of the vasculature using autologous Y-shaped subcutaneous veins. This reconstruction had no complications in any cases. This procedure has not been reported in uterine transplantation but was recently mentioned in the context of kidney transplantation [17]. Although the anatomy of the canine uterus differs distinctly from the human anatomy, the results of this study supply information regarding the anatomy of the canine uterus and immunosuppression during the transplantation process. This model could be helpful in uterine transplantation training and may contribute to improved transplantation outcomes in humans.
In this study, we examined the possibility of a living dog model for uterine transplantation, which had not been done previously [5-8]. The mean organ procurement time was 75.6 minutes, and a microscope was used to minimize damage to the fragile canine small uterine vessels. This step can be performed in humans with surgical loupes, as uterine vessel diameters are 2–3 mm compared to 1–2 mm in canine samples. In the first case of uterine transplant, by Brannstrom et al. [1], the duration of living donor surgery was 10–13 hours, similar to the first experiment in Germany [18]. However, the duration of living donor surgery in uterine transplantation was shortened to 6–8 hours in a Czech trial [19]. The deceased donor model of uterine transplantation was developed several decades ago, with a technique for isolating the bicornuate dog uterus and adnexa with blood vessels for re-anastomosis [5]. The standard portion of the internal iliac artery was dissected from its origin and freed caudally with ligations of all branches except the uterine and superior vesical arteries. The uterine veins were dissected cranially from the uterus to fuse the internal and external iliac veins. The donor animals were sacrificed, since the vascular pedicles and common iliac artery were removed. The present study of a canine uterine donor model showed a relatively short time of organ procurement, and all donors were living.
Previous research has indicated that the first warm ischemic period, occurring at body temperature, is more harmful than the second warm ischemic period, marked by a gradual increase in temperature and protection with preservation solution [20]. In the first publication on uterus isolation [5], flushing was performed with heparinized saline through the common iliac artery of the specimen with 30 minutes of warm ischemia. Another report involved the use of normal thermal heparinized saline until all traces of blood had been washed away with 45 minutes of warm ischemia [7]. In the present study, the first warming period was relatively short (3.5 minutes), which could reduce the period of harm to the body. The cold ischemia time most commonly ranged from 41 to 50 minutes, including the time for angioplasty, in which the lengths of uterine vessels were augmented on both sides with subcutaneous veins. Even so, the recipient operation time was comparable to previous studies [21,22]. In our new living uterine donor, the short operation time helped reduce the time of blood leaking and loss; this could be very important for the survival of the recipient canine, since a previous report indicated that this was a main complication leading to recipient death [23].
Our operation method showed efficiency in reducing the dissection time and decreasing the tension on the uterus. Importantly, our method did not affect the urinary tract; this was crucial because the ureter and bladder are very fragile, as supported by previous research indicating main complications of bladder devascularization, injury, and ureteral devascularization in both animal models [3] and humans [1,24].
In uterine transplantation, it is crucial to ensure adequate vascular anastomosis sites and uterine structural support [25]. Vascular anastomosis in dog utero-tubal-ovarian transplantation has primarily involved end-to-end anastomosis of the internal iliac artery and end-to-side anastomosis of the internal iliac veins [5,7,8]. In most of those studies, the viability of the uterus after transplantation was not evaluated, and the outcome could not be determined. In some studies, researchers have examined omentopexy for vascularization and demonstrated viable uterine tissue. However, in a comparative study, all uteri in the omentopexy group had degenerated by 3 months after surgery, while those transplanted via vascular anastomosis were still viable [6]. Our study showed anastomosis patency, a clear pulse, and no sign of necrosis of the transplanted uterus, demonstrating that vascular anastomosis was viable. Additionally, we decided not to anastomose the ovarian arteries because of their extremely small size (only 0.3 to 0.5 mm). In this situation, the ovaries would not be included since, based on previous findings, these would have been necrosed [5,8].
Despite the results, this study still had several limitations, including a lack of histological examination to evaluate the transplanted uterine structure and status. Additionally, observations, including the X-ray imaging on day 5 after uterine transplantation and the survival rate at 3 hours after transplantation, were certainly short-term and insufficient for complete follow-up. Nevertheless, in this study, we successfully verified the uterine anatomy and introduced a new canine living-donor uterine transplantation model for uterine transplantation training in Vietnam.
The anatomic features of the canine uterus differed substantially from those of the human uterus, with the uterine vessels originating from vaginal vessels, which were branches of pudendal vessels. The diameter of the uterine vascular pedicle was small (1 to 1.5 mm for arteries and 1.2 to 2.0 mm for veins), necessitating manipulation under a microscope. The living-donor uterine transplantation model constructed in this study was feasible, with the transplanted uterus surviving in most cases (13 of 15).
ACKNOWLEDGMENTS
Author Contributions
Conceptualization: XHD, THT. Data curation: XHD, THT. Formal analysis: TCN, TAN, MTTT. Investigation: XHD, THT, TCN. Methodology: TCN, TAN, MTTT. Visualization: XHD. Writing–original draft: XHD, THT. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Brannstrom M, Johannesson L, Dahm-Kahler P, Enskog A, Molne J, Kvarnstrom N, et al. 2014; First clinical uterus transplantation trial: a six-month report. Fertil Steril. 101:1228–36. DOI: 10.1016/j.fertnstert.2014.02.024. PMID: 24582522.

2. Brannstrom M, Johannesson L, Bokstrom H, Kvarnstrom N, Molne J, Dahm-Kahler P, et al. 2015; Livebirth after uterus transplantation. Lancet. 385:607–16. DOI: 10.1016/S0140-6736(14)61728-1. PMID: 25301505.

3. Favre-Inhofer A, Carbonnel M, Domert J, Cornet N, Chastant S, Coscas R, et al. 2022; Involving animal models in uterine transplantation. Front Surg. 9:830826. DOI: 10.3389/fsurg.2022.830826. PMID: 35284480. PMCID: PMC8904568.

4. Zhordania IF, Gotsiridze OA. 1964; Vital activity of the excised uterus and its appendages after their autotransplantation into omentum. Experimental research. Acta Chir Plast. 6:23–32.
5. Eraslan S, Hamernik RJ, Hardy JD. 1966; Replantation of uterus and ovaries in dogs, with successful pregnancy. Arch Surg. 92:9–12. DOI: 10.1001/archsurg.1966.01320190011002. PMID: 5948103.

6. Barzilai A, Paldi E, Gal D, Hampel N. 1973; Autotransplantation of the uterus and ovaries in dogs. Isr J Med Sci. 9:49–52.
7. Truta E, Pop I, Popa D, Ionescu M, Truta F. 1969; Experimental re- and transplantation of the internal female genital organs. Rom Med Rev. 13:53–8.
8. Yonemoto RH, Du Sold WD, Deliman RM. 1969; Homotransplantation of uterus and ovaries in dogs: a preliminary report. Am J Obstet Gynecol. 104:1143–51. DOI: 10.1016/S0002-9378(16)34288-0. PMID: 5799618.
9. Wall AE, Testa G, Axelrod D, Johannesson L. 2021; Uterus transplantation-questions and answers about the procedure that is expanding the field of solid organ transplantation. Proc (Bayl Univ Med Cent). 34:581–5. DOI: 10.1080/08998280.2021.1925064. PMID: 34456477. PMCID: PMC8366946.

10. Ozkan O, Dogan NU, Ozkan O, Mendilcioglu I, Dogan S, Aydinuraz B, et al. 2016; Uterus transplantation: from animal models through the first heart beating pregnancy to the first human live birth. Womens Health (Lond). 12:442–9. DOI: 10.1177/1745505716653849. PMID: 27638900. PMCID: PMC5373276.

11. Racho El-Akouri R, Kurlberg G, Brännström M. 2003; Successful uterine transplantation in the mouse: pregnancy and post-natal development of offspring. Hum Reprod. 18:2018–23. DOI: 10.1093/humrep/deg396. PMID: 14507815.

12. Groth K, Brannstrom M, Molne J, Wranning CA. 2010; Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 25:697–704. DOI: 10.1093/humrep/dep470. PMID: 20085916.

13. Wranning CA, El-Akouri RR, Lundmark C, Dahm-Kahler P, Molne J, Enskog A, et al. 2006; Auto-transplantation of the uterus in the domestic pig (Sus scrofa): surgical technique and early reperfusion events. J Obstet Gynaecol Res. 32:358–67. DOI: 10.1111/j.1447-0756.2006.00426.x. PMID: 16882260.

14. Wranning CA, Marcickiewicz J, Enskog A, Dahm-Kahler P, Hanafy A, Brännström M. 2010; Fertility after autologous ovine uterine-tubal-ovarian transplantation by vascular anastomosis to the external iliac vessels. Hum Reprod. 25:1973–9. DOI: 10.1093/humrep/deq130. PMID: 20519245.

15. El-Akouri RR, Kurlberg G, Dindelegan G, Molne J, Wallin A, Brannstrom M. 2002; Heterotopic uterine transplantation by vascular anastomosis in the mouse. J Endocrinol. 174:157–66. DOI: 10.1677/joe.0.1740157. PMID: 12176655.

16. Wranning CA, Akhi SN, Diaz-Garcia C, Brannstrom M. 2011; Pregnancy after syngeneic uterus transplantation and spontaneous mating in the rat. Hum Reprod. 26:553–8. DOI: 10.1093/humrep/deq358. PMID: 21159686.

17. Miyauchi Y, Noda T, Miura N, Kikugawa T, Saika T. 2021; Venous reconstruction using a Y-shaped saphenous vein in kidney transplantation: a report of three cases. IJU Case Rep. 4:146–9. DOI: 10.1002/iju5.12266. PMID: 33977243. PMCID: PMC8088897.

18. Brucker SY, Brannstrom M, Taran FA, Nadalin S, Konigsrainer A, Rall K, et al. 2018; Selecting living donors for uterus transplantation: lessons learned from two transplantations resulting in menstrual functionality and another attempt, aborted after organ retrieval. Arch Gynecol Obstet. 297:675–84. DOI: 10.1007/s00404-017-4626-z. PMID: 29270725.

19. Chmel R, Novackova M, Janousek L, Matecha J, Pastor Z, Maluskova J, et al. 2019; Revaluation and lessons learned from the first 9 cases of a Czech uterus transplantation trial: four deceased donor and 5 living donor uterus transplantations. Am J Transplant. 19:855–64. DOI: 10.1111/ajt.15096. PMID: 30151893.

20. Feuillu B, Cormier L, Frimat L, Kessler M, Amrani M, Mangin P, et al. 2003; Kidney warming during transplantation. Transpl Int. 16:307–12. DOI: 10.1111/j.1432-2277.2003.tb00305.x. PMID: 12759721.

21. Dion L, Le Lous M, Nyangoh Timoh K, Levêque J, Arnaud A, Henri-Malbert C, et al. 2021; Single bilateral ovarian venous return in uterine transplant: validation in an orthotopic auto-transplant model in the Yucatan minipig. J Gynecol Obstet Hum Reprod. 50:102059. DOI: 10.1016/j.jogoh.2021.102059. PMID: 33421624.

22. Andraus W, Ejzenberg D, Santos RM, Mendes LR, Arantes RM, Baracat EC, et al. 2017; Sheep model for uterine transplantation: the best option before starting a human program. Clinics (Sao Paulo). 72:178–82. DOI: 10.6061/clinics/2017(03)08.

23. Favre-Inhofer A, Carbonnel M, Revaux A, Sandra O, Mougenot V, Bosc R, et al. 2018; Critical steps for initiating an animal uterine transplantation model in sheep: experience from a case series. Int J Surg. 60:245–51. DOI: 10.1016/j.ijsu.2018.11.017. PMID: 30481612.

24. Fageeh W, Raffa H, Jabbad H, Marzouki A. 2002; Transplantation of the human uterus. Int J Gynaecol Obstet. 76:245–51. DOI: 10.1016/S0020-7292(01)00597-5. PMID: 11880127.

25. Mäkikallio K, Tekay A, Jouppila P. 2004; Uteroplacental hemodynamics during early human pregnancy: a longitudinal study. Gynecol Obstet Invest. 58:49–54. DOI: 10.1159/000077914. PMID: 15087597.

Fig. 2
Organ procurement. (A) Infusion of 4 °C Ringer lactate solution with 2% lidocaine into arteries (blue needle) and veins (yellow needle) until the water was clear and the uterus was white and pink, then arterial infusion with 4 °C Custodiol (Dr. Franz Köhler Chemie GmbH) solution. (B) Organ procurement time for each donor dog.
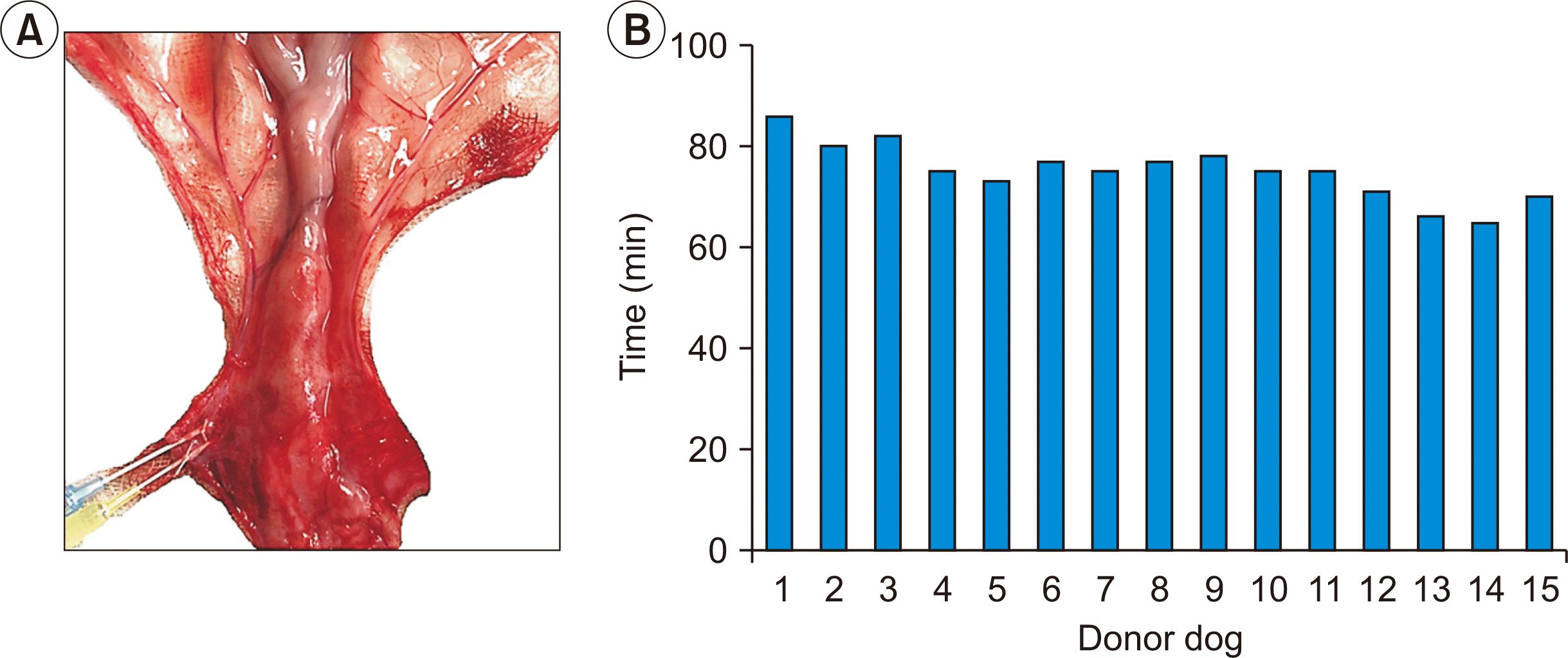
Fig. 3
Donor uterus and venous graft for augmentation of the vascular pedicle. (A) Experimental canine donor uterus graft model with arterial Y-graft. (B) Donor’s venous graft under ×10 magnification. Arrow: Y-graft anatomoses; 1 and 2: the two branches of the Y-graft; 3: mesometrium. (C) Length of the graft pedicle after angioplasty.
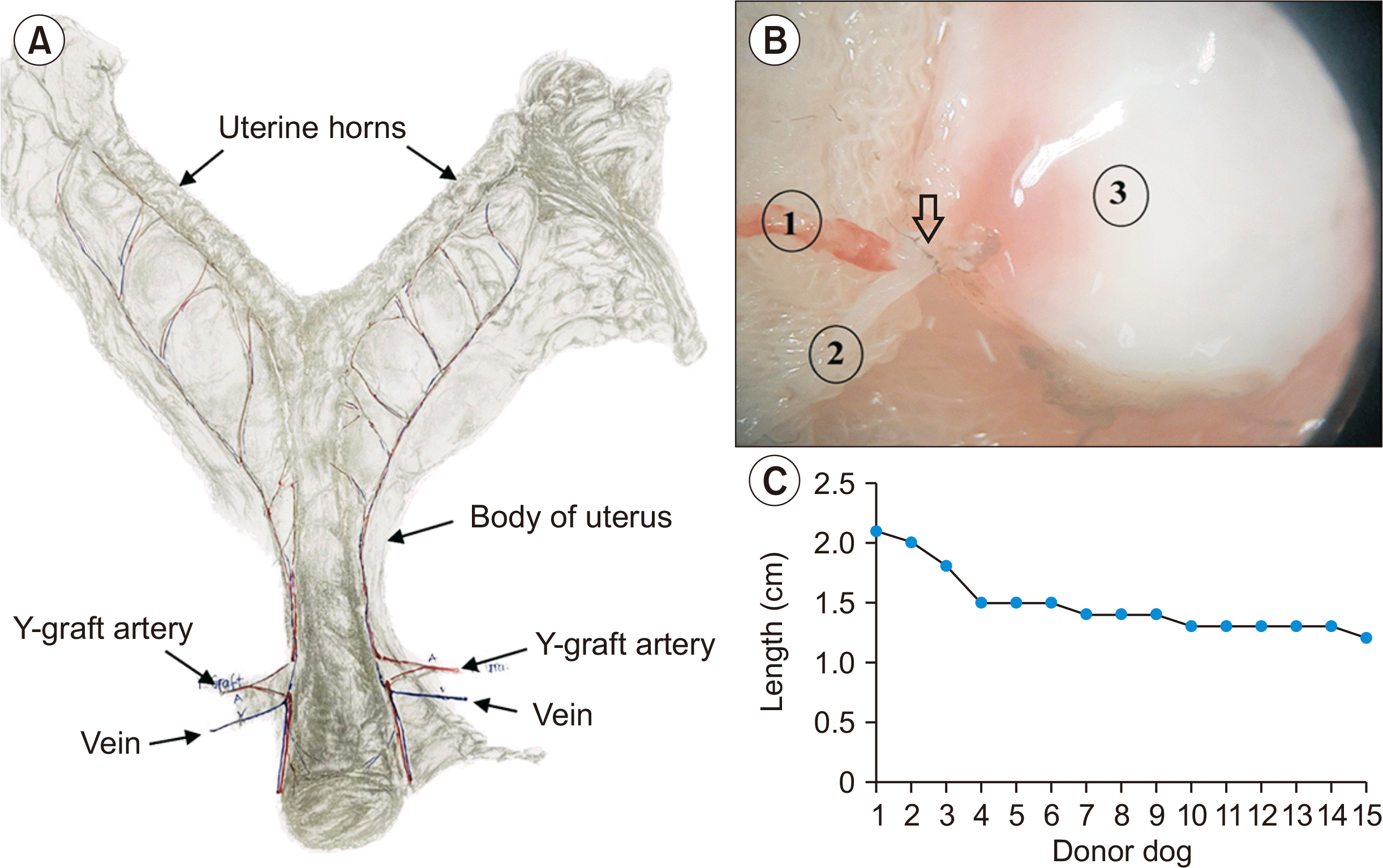
Fig. 4
Operative times in minutes (first warm ischemic time, cold ischemic time, and duration of implantation) for each operated dog.
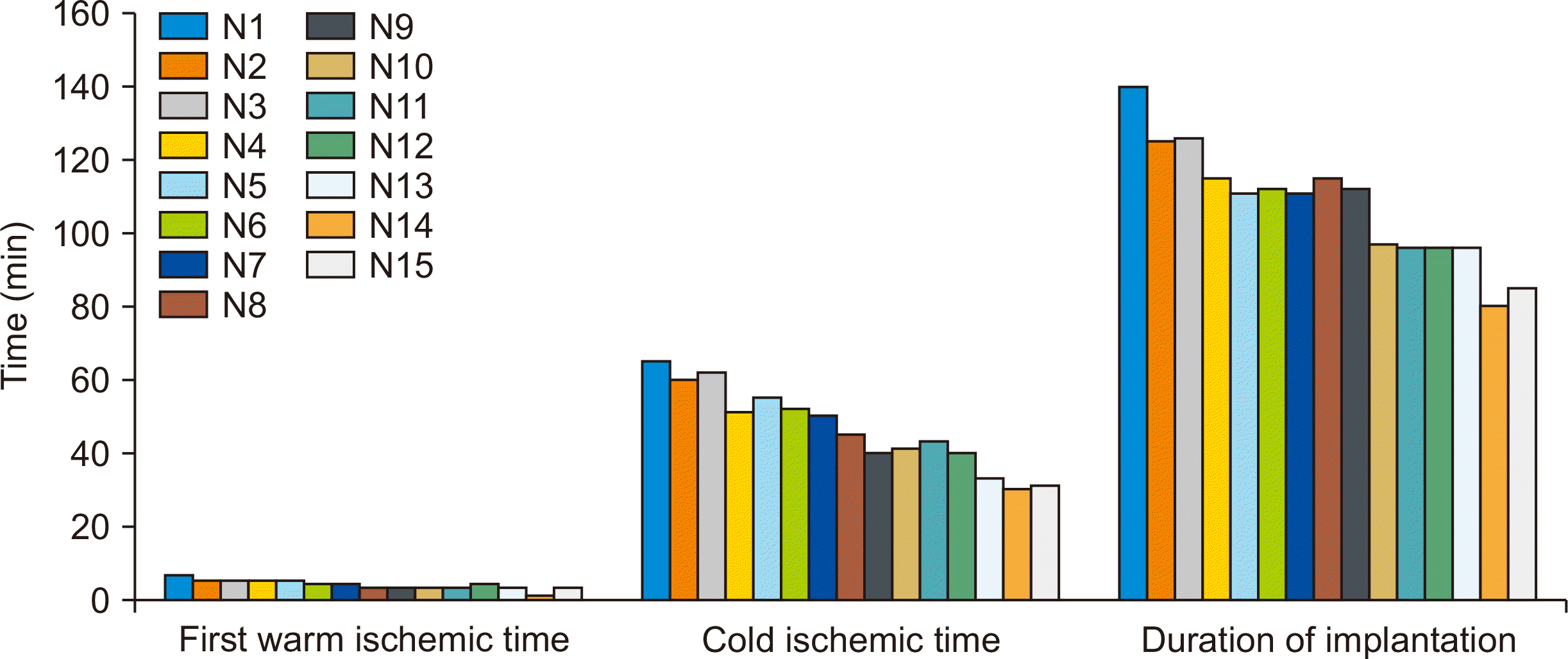
Fig. 5
(A) X-ray image after uterus transplant on day 5. (B) Images of contrast enhancement of the transplanted canine uterus after 15 minutes.
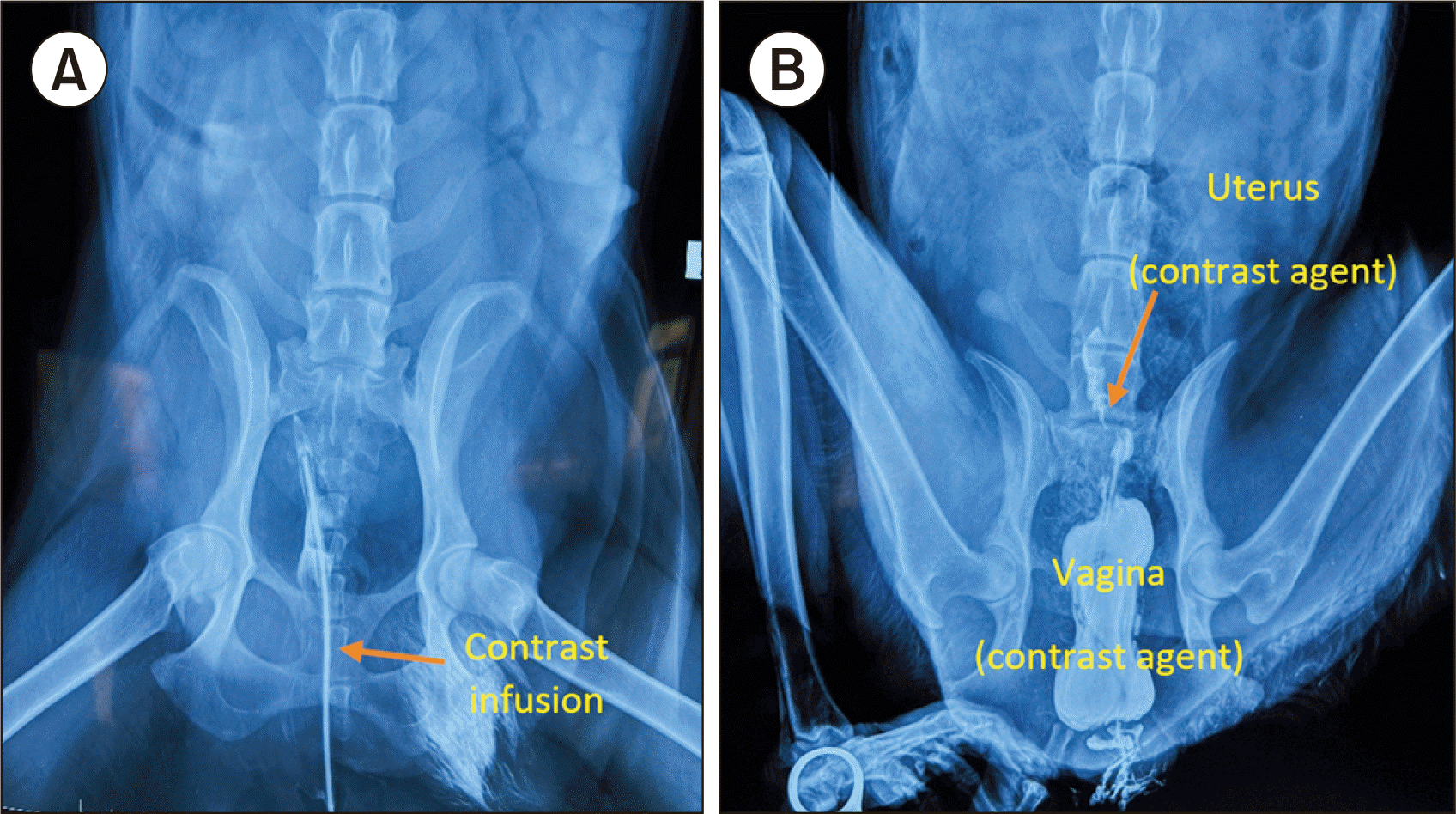
Table 1
Uterus and uterine blood vessel anatomic characteristics




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download