CASE REPORT
Ethical approval requirement was waived by the institutional ethical approval board, considering that this is a case report. Written informed consent from the patient has been taken for the publication of this case report.
A 61-year-old female RTR with hypertension, irritable bowel syndrome, and a distant history of pulmonary tuberculosis presented to our center in May 2021 with symptoms of fever, chills, rigors, dysuria, nausea, vomiting, and oliguria of 3 days’ duration. She had undergone living donor renal transplantation in January 2011 at our center, with her husband as the donor. She was on immunosuppression with azathioprine (100 mg, once daily), tacrolimus (1.5 mg, twice daily) and prednisolone (7.5 mg, once daily), all taken orally. She had been managed conservatively for recurrent urinary tract infections (UTIs) since 2016, with culture-based antibiotics (the organisms isolated were Escherichia coli and Klebsiella pneumoniae) and was on long-term prophylaxis with trimethoprim (160 mg) and sulfamethoxazole (800 mg) as a nightly dose. The patient was receiving routine follow-up, including annual ultrasonography (USG) of the abdomen, and her previous USG done 6 months before was essentially normal.
Her baseline serum creatinine level was 1.3 mg/dL, which had risen to 1.8 mg/dL. Noncontrast computed tomography and magnetic resonance imaging (MRI) of the abdomen were suggestive of a 13×8 mm heterogeneously hyperdense nodular lesion (65 Hounsfield units) in the proximal TU, just below the ureteropelvic junction (UPJ), causing hydronephrosis of the intrarenal pelvis and acute pyelonephritis of the transplant kidney (TK) (
Fig. 1). On cystoscopy and retrograde pyelography (RGP), contrast was not visualized beyond the distal TU. Retrograde double-J (DJ) stenting of the TU was done with difficulty, and pyelonephritis was managed with antibiotics. One month later, cystoscopy and RGP (done after passing ureteric catheter over a guidewire beyond the ureteric obstruction) revealed a cut-off of contrast flow just below the UPJ (
Fig. 2A and B). Ureteroscopy revealed unhealthy tissue at the proximal TU (
Fig. 2C), and the tiny biopsy obtained was interpreted as high-grade UC. Whole-body fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a metabolically active lesion in the proximal TU, measuring 14×18×22 mm, with no other lesion elsewhere.
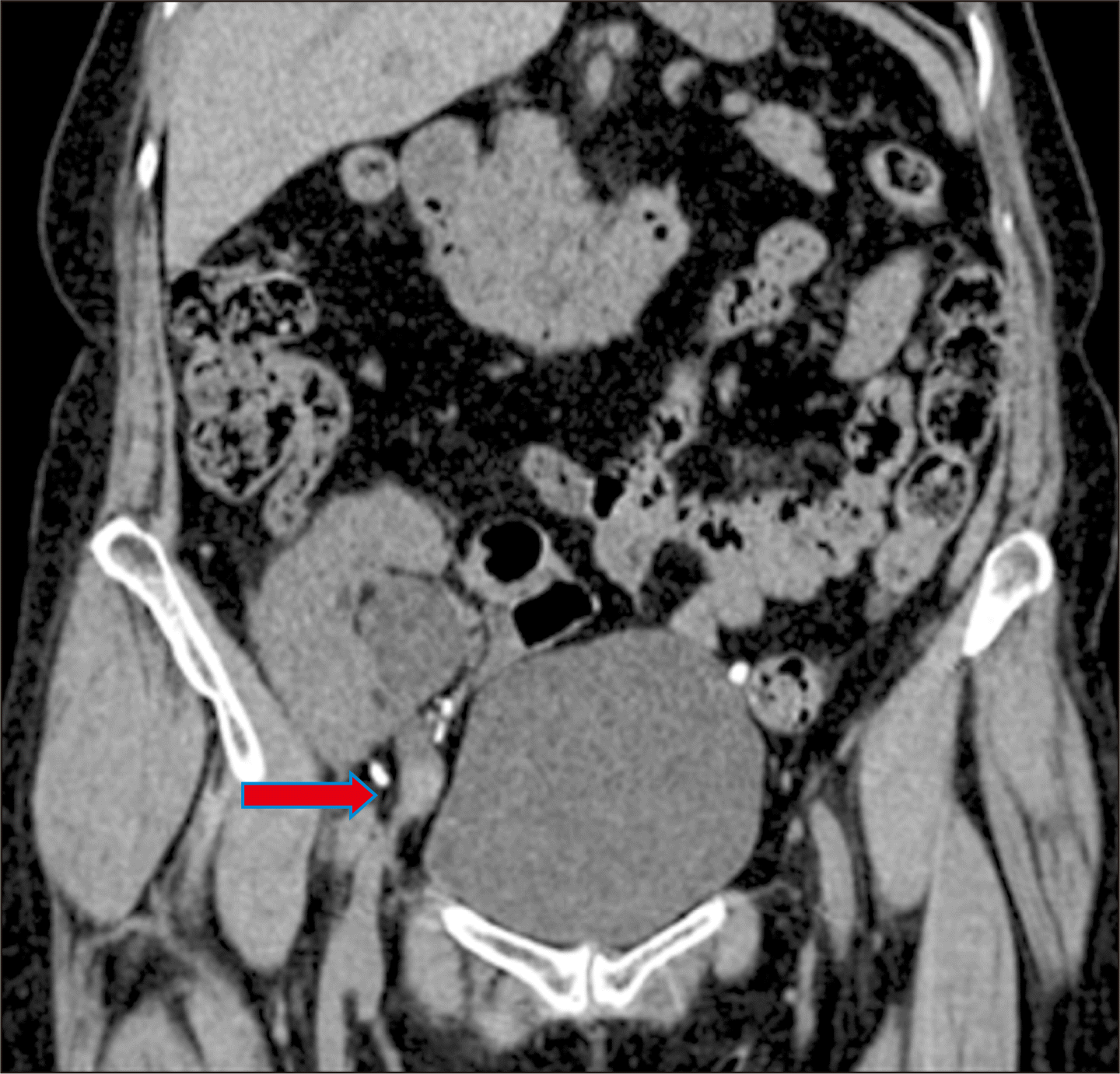 | Fig. 1Noncontrast computed tomography of the kidneys, ureters, and bladder scan showing hydronephrosis of the transplant kidney with the intrarenal pelvis and a lesion in the proximal part of the graft ureter, just below the ureteropelvic junction (red arrow). 
|
 | Fig. 2(A) Retrograde pyelography showing hydronephrosis of the transplant kidney with a cut-off of contrast just below the ureteropelvic junction. (B) The ureteroscope was not able to negotiate beyond the site of obstruction, and (C) ureteroscopy showing unhealthy tissue (yellow arrow). 
|
With the aim of treating the malignancy and saving the TK, the patient underwent total ureterectomy of the TU along with bladder cuff excision and pyelovesicostomy. Intraoperatively, there were dense adhesions around the TK and TU. The proximal margin was initially positive on a frozen section and it was revised twice further at the intrarenal pelvis to achieve negative margins, and the bladder was mobilized with division of left superior vesical pedicle and pyelovesicostomy completed (
Fig. 3A and B). The serum creatinine level returned to 1.2 mg/dL by the postoperative day (POD) 7. The initial anastomotic urine leakage through the abdominal drain, stopped on the POD 12. The patient was discharged with a per-urethral catheter (PUC) after drain removal on the POD 16. The PUC and the DJ stent were removed on POD 28 and 35, respectively.
 | Fig. 3Intraoperative images showing (A) dissection of the transplant ureter, (B) completed pyelovesicostomy with the bladder distended with saline to check for urine leak at the anastomosis, and (C) gross photograph of the cut-open resected specimen of ureterectomy with a whitish, firm infiltrative tumor present at the proximal end (red arrow). 
|
The final histopathology of the specimen revealed a tumor in the proximal 3 cm of the TU (total TU length, 7 cm) (
Fig. 3C), causing partial obliteration of the ureteric lumen. The tumor was arising from the urothelial mucosa and showed a mixed pattern comprising areas with a villous and glandular pattern (90%), along with foci of reticulocystic architecture (10%). It was breaching the muscularis, but did not extend beyond it (
Fig. 4). No area of conventional high-grade UC was identified despite sampling the tumor in its entirety. The tumor cells were immunopositive for CK7, cancer antigen (CA19.9), and GATA3 and negative for CK20 and CDX2 (
Fig. 5). Additional immune-markers revealed positivity for glypican-3 and alpha-fetoprotein (AFP) in the areas with reticulocystic architecture, and a mutated p53 phenotype. The final pathological diagnosis was primary adenocarcinoma (villoglandular type) with YSD of the TU, pT2NxMx.
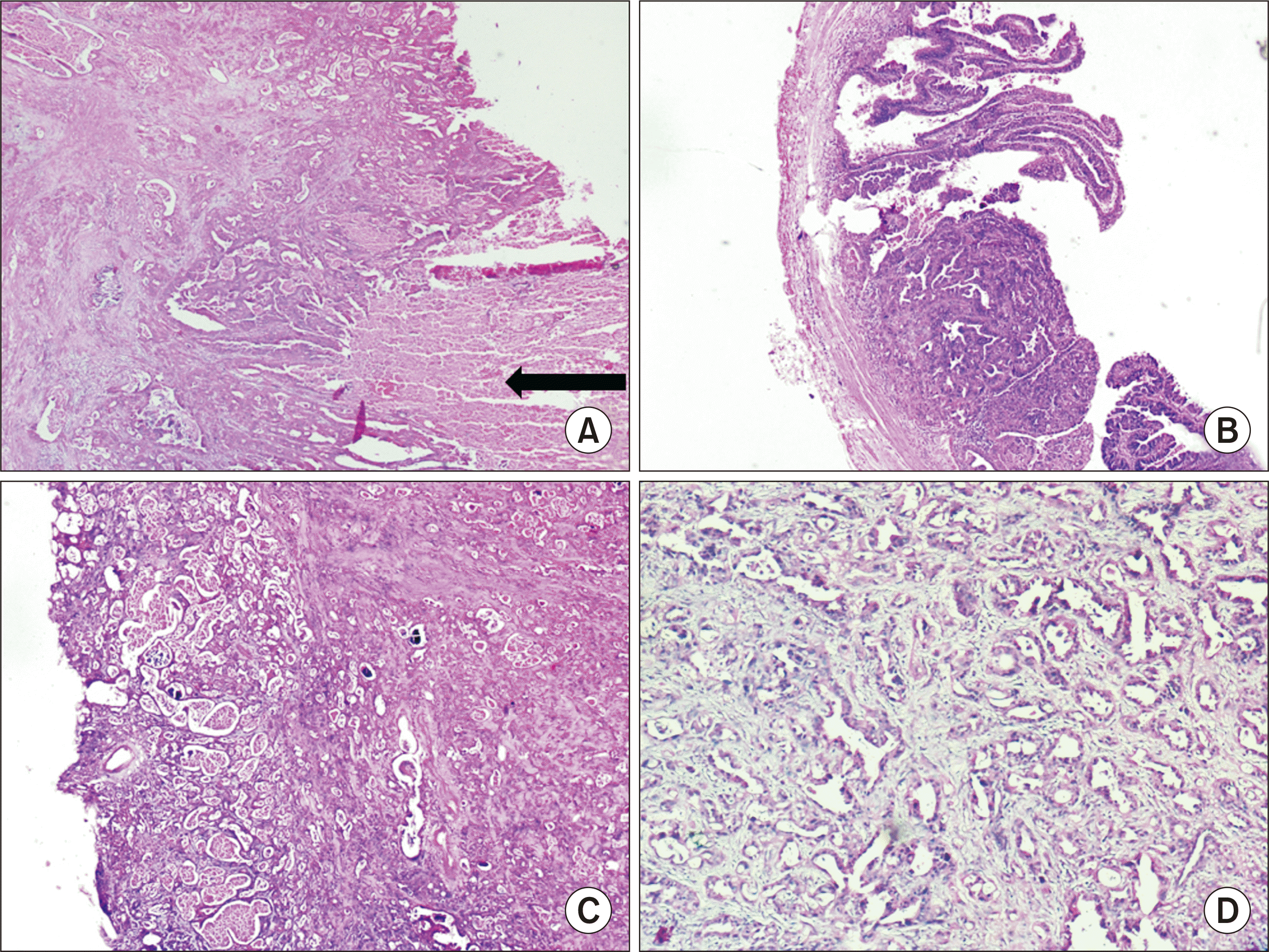 | Fig. 4H&E stained histopathological images of the tumor: (A) tumor composed of areas of villoglandular morphology infiltrating the underlying stroma along with areas of necrosis (black arrow; H&E, ×40), (B) another area of the ureteric tumor with the lining showing a well-defined villoglandular architecture (H&E, ×40), (C) tumor with areas showing a reticulocystic pattern, some of the cystic areas containing secretions within (H&E, ×40), and (D) higher-power view of another area with glandular differentiation, showing distorted glands lined by highly pleomorphic cells (H&E, ×100). 
|
 | Fig. 5Immunohistochemistry characterizing the lesion as a ureteric adenocarcinoma with a villoglandular pattern and areas of yolk sac differentiation: (A) tumor cells are diffusely positive for CK7 (×100), (B) GATA 3 (×100), and (C) CA 19.9 (×100). (D) The reticulocystic areas were positive for alpha-fetoprotein (×200) and (E) glypican 3 (×200). (F) The tumor had a mutated phenotype for p53 (×100). 
|
The decision was made for the patient to receive regular follow-up, with no adjuvant therapy. Postoperatively her immunosuppression regime was modified to enteric coated mycophenolate sodium (180 mg, twice daily), tacrolimus (1.5 mg, twice daily), and prednisolone (7.5 mg, once daily); azathioprine was stopped. MRI scans 3 and 12 months after the definitive surgery revealed a patent anastomosis between the transplanted renal pelvis and the right superolateral wall of bladder; there was no recurrent lesion or hydronephrosis (
Fig. 6). Cystoscopy performed 6 months after surgery also confirmed the same findings. The patient is receiving regular follow-up with a nephrologist, and repeated USG also revealed no hydronephrosis or tumor recurrence.
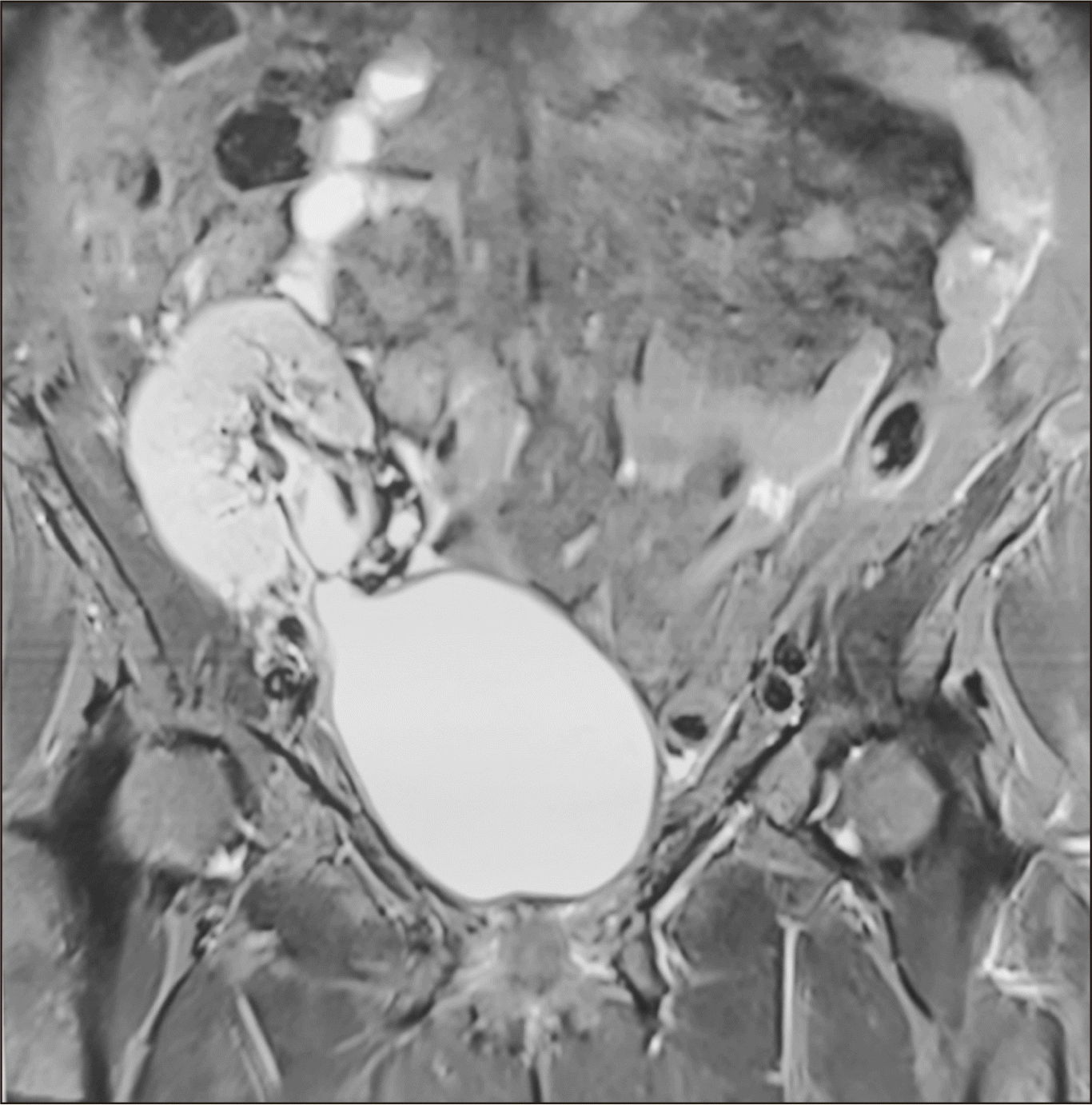 | Fig. 6Postoperative magnetic resonance imaging of the abdomen, 12 months after total transplant ureterectomy and pyelovesicostomy, showing a patent anastomosis between the transplanted renal pelvis and the superolateral wall of the bladder, with no lesion in the renal pelvis or bladder and significant improvement of hydronephrosis. 
|
The male spousal donor was evaluated for any tumor origin from the donor. His genitourinary and rectal examination, abdominal imaging, and pelvic imaging were normal. All tumor markers, such as lactate dehydrogenase, AFP, prostate-specific antigen, carcinoembryonic antigen, and CA19.9, were normal.
Go to :

DISCUSSION
In a study from South Korea, among 2,186 RTRs, bladder UC occurred in five patients and upper tract UC in nine patients—eight in the native upper tract and one in the transplanted renal pelvis [
1]. Olsburgh et al. [
2] described four cases of upper tract UC of the TU, occurring 11–31 years after transplantation. Their literature review revealed a total of 33 cases of upper tract UC, including their four, between 1995 and 2016. Out of these 33 cases, only five patients underwent resection and reconstruction, similar to our patient; the rest underwent nephroureterectomy of TK and TU or the treatment was unknown. We even obtained consent for graft nephroureterectomy, which would have been a necessity if we had not achieved negative proximal margins. We proceeded with the conservative surgery approach, since we wanted to save the functioning graft kidney; moreover, there was no periureteric or lymph node involvement on imaging.
The final histopathology was surprising, as the report indicated an adenocarcinoma with YSD. The absence of areas of conventional UC made the tumor a pure adenocarcinoma of the ureter, an extremely rare histopathological subtype. The immunopositivity for GATA3 delineated its urothelial origin and corroborated YSD. Pure adenocarcinomas of the urothelium are rare, accounting for <1% of all cancers of the renal pelvis and ureter [
3]. Adenocarcinomas of the renal pelvis and ureter are often associated with chronic obstruction, inflammation, infection, and urolithiasis [
4]. Recurrent UTIs with the possibility of reflux into the TU may have been a predisposing factor in our patient. Clear cell renal cell carcinoma of the native left kidney and adenocarcinoma of the ipsilateral ureter, associated with urolithiasis, has been reported in an RTR [
5]. A case of endometrioid adenocarcinoma in the native left mid-ureter of a postmenopausal RTR has also been reported [
6]. The patient had a history of total abdominal hysterectomy and bilateral salpingo-oophorectomy 14 years prior and had since been on unopposed estrogen replacement therapy. A case of adenocarcinoma of right distal ureter has been reported in a 62-year-old male with previous urolithiasis [
7]. The patient received external beam radiation to the postoperative bed and lymph nodes, since he had positive distal margins after laparoscopic right radical nephroureterectomy. Since our patient was an RTR on immunosuppression and with no clear evidence for adjuvant treatment in adenocarcinoma [
7], we decided to only perform follow-up.
YSD has not yet been recognized as a separate subtype of UC by the World Health Organization; however, an extensive literature search revealed a few case reports [
8,
9]. YSD has been reported in several other somatic malignancies, including those at gastrointestinal [
10,
11] and gynecological sites [
12,
13]. The possibility of a metastatic tumor was highly suspected in our case due to the unusual histomorphology, but ruled out by FDG-PET scan. Moreover, the intraluminal location of the tumor helped ascertain that the ureteric mucosa was the primary site of origin. Correlation with serum AFP levels was not helpful as the diagnosis was established after resection of the entire tumor.
Since the patient had irritable bowel syndrome, she was initially on azathioprine, considering that mycophenolate mofetil causes upper and lower gastrointestinal side effects. After the diagnosis of malignancy, azathioprine was stopped since it is known to increase the risk of urinary tract cancers [
14]. She was started on enteric coated mycophenolate sodium to avoid aggravating her irritable bowel syndrome.
This case report highlights the rare possibility of an extremely uncommon histopathological subtype of malignancy occurring in the TU in the setting of recurrent UTIs. With the therapeutic dilemma of choosing between sound oncological treatment and saving the TK, an individualized approach is required in such unique cases.
Go to :









 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download