This article has been
cited by other articles in ScienceCentral.
Abstract
Kaposi’s sarcoma (KS) is a disease that is not widely known among the general public, but has a high prevalence among organ transplant recipients. Here, we present a rare case of intragraft KS after kidney transplantation. A 53-year-old woman who had been on hemodialysis due to diabetic nephropathy underwent deceased-donor kidney transplantation on December 7, 2021. Approximately 10 weeks after kidney transplantation, her creatinine level increased to 2.99 mg/dL. Upon examination, ureter kinking was confirmed between the ureter orifices and the transplanted kidney. As a result, percutaneous nephrostomy was performed, and a ureteral stent was inserted. During the procedure, bleeding occurred due to a renal artery branch injury, and embolization was performed immediately. Subsequently, kidney necrosis and uncontrolled fever developed, leading to graftectomy. Surgical findings revealed that the kidney parenchyma was necrotic as a whole, and lymphoproliferative lesions had formed diffusely around the iliac artery. These lesions were removed during graftectomy, and a histological examination was performed. The kidney graft and lymphoproliferative lesions were diagnosed as KS based on a histological examination. We report a rare case in which a recipient developed KS in the kidney allograft as well as in adjacent lymph nodes.
Go to :

Keywords: Kidney transplantation, Kaposi’s sarcoma, Maligancy after transplantation, Case report
|
HIGHLIGHTS |
This was a rare case in which Kaposi’s sarcoma (KS) occurred in an allograft, necessitating graftectomy. KS typically appears in skin lesions, but can also occur in lymph nodes, lungs, or the gastrointestinal tract. We report a rare case in which a recipient developed KS in a kidney allograft and adjacent lymph nodes.
|
Go to :

INTRODUCTION
Kaposi’s sarcoma (KS) is a malignancy that most commonly affects organ transplant recipients and individuals living with HIV/AIDS (human immunodeficiency virus/ acquired immunodeficiency syndrome). Among transplant recipients, the average time to diagnosis after transplantation is approximately 12 months [
1]. KS usually manifests as brown spots on the skin and mucous membranes, such as in the mouth and esophagus. It often appears on the extremities without accompanying symptoms (e.g., pain or discharge); if it occurs in the gastrointestinal tract or lungs, it may cause symptoms such as gastrointestinal bleeding and respiratory distress. Lesions that can be easily identified with the naked eye can be confirmed through simple histological examination. Tumor regression can be achieved by reducing the dosage of immunosuppressive drugs taken after organ transplantation and using mammalian target of rapamycin (mTOR) inhibitors [
2]. Here, we report a rare case of KS occurring in an allograft within a notably short period, just 10 weeks after kidney transplantation.
Go to :

CASE REPORT
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-0101). Written informed consent was obtained from the patient.
A 53-year-old woman, who had been on hemodialysis due to diabetic nephropathy, received a deceased-donor kidney transplant on December 7, 2021. The blood type of the recipient and the donor was O, and there was no human leukocyte antigen mismatch. The immunosuppressive treatment regimen included tacrolimus (2.25 mg twice daily), mycophenolate mofetil (500 mg twice daily), and intravenous methylprednisolone (500 mg), which was gradually tapered down to 1.5 tablets once daily. The serological test for HIV was negative, and a human herpesvirus 8 (HHV-8) test was not performed. The patient was hospitalized when her serum creatinine level increased to 2.99 mg/dL at 10 weeks after the kidney transplantation. The biopsy findings at that time were non-specific, but a renal scan showed a possible acute partial obstruction between the right transplanted kidney and the ureteral orifice (
Fig. 1). Ureteral kinking was confirmed, and percutaneous nephrostomy and anterograde double-J stent insertion were performed (
Fig. 2).
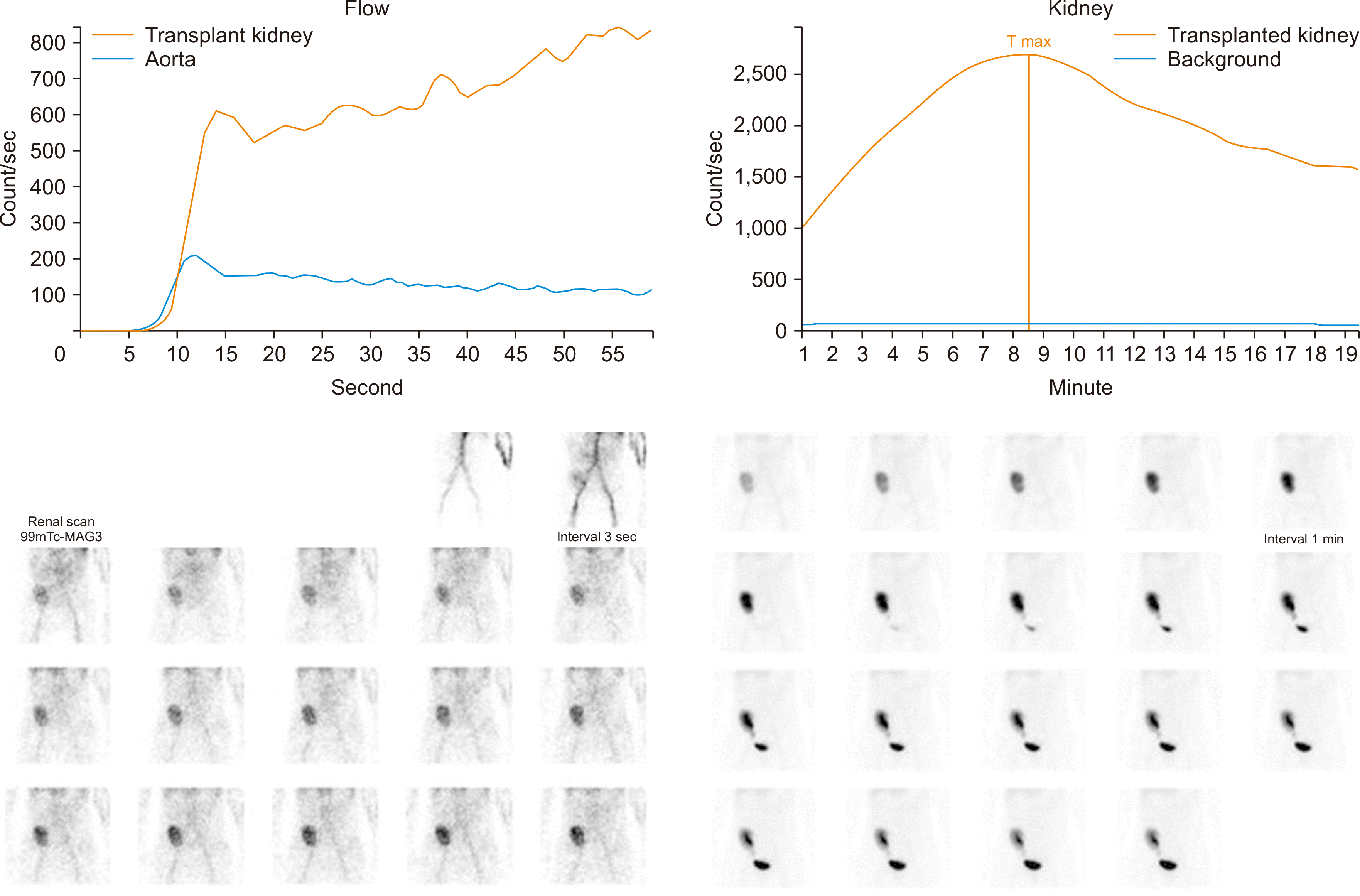 | Fig. 1Possible acute partial obstruction on a renal scan. The perfusion and uptake of the transplanted kidney are mildly decreased, and there is activity migrating to the bladder seen in the 6–7-minutes images. Additionally, there is mild pelvocalyceal and parenchymal retention activity. 
|
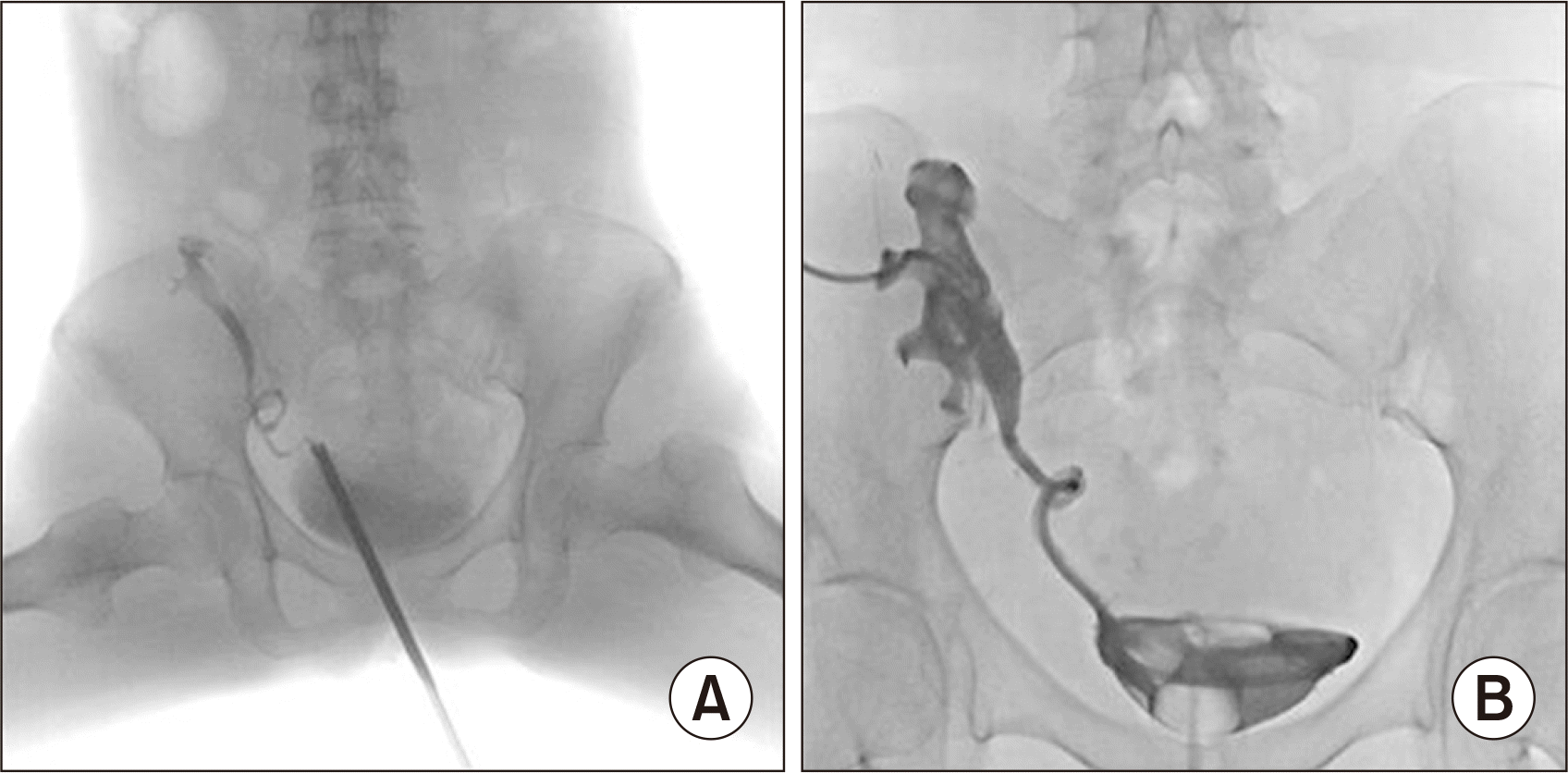 | Fig. 2(A) Ureteral kinking confirmed on retrograde pyelography. (B) Percutaneous nephrostomy and anterograde double-J stent insertion. 
|
Follow-up abdominopelvic computed tomography (CT) revealed hematoma formation in the bladder, renal pelvis, and ureter, along with obstructive nephropathy in the transplanted kidney. There were also multiple enlarged retroperitoneal lymph nodes, which were initially deemed unremarkable because lymphadenopathy had previously been found. Active bleeding was confirmed at the percutaneous nephrostomy insertion site, which explained the multiple hematomas. Therefore, selective embolization was performed, after which no abnormalities were detected by Doppler ultrasonography to assess the vascular distribution. Postembolization CT revealed embolic material, but it was indistinguishable from the previous hematomas, and there was no evidence of active bleeding. However, the previously enlarged retroperitoneal lymph nodes had become more prominent.
Approximately 1 week later, a renal scan revealed complete obstruction of the transplanted kidney. Postembolization CT showed necrosis of the grafted kidney (
Fig. 3). A follow-up evaluation after 1 week revealed unfavorable findings, including an increase in the leukocyte count from 5.2 × 10
3/μL to 32.5 × 10
3/μL, an increase in the C-reactive protein level from 4.08 mg/dL to 10.97 mg/dL, and an uncontrolled fever of above 38 °C. These manifestations necessitated graftectomy, which was subsequently performed.
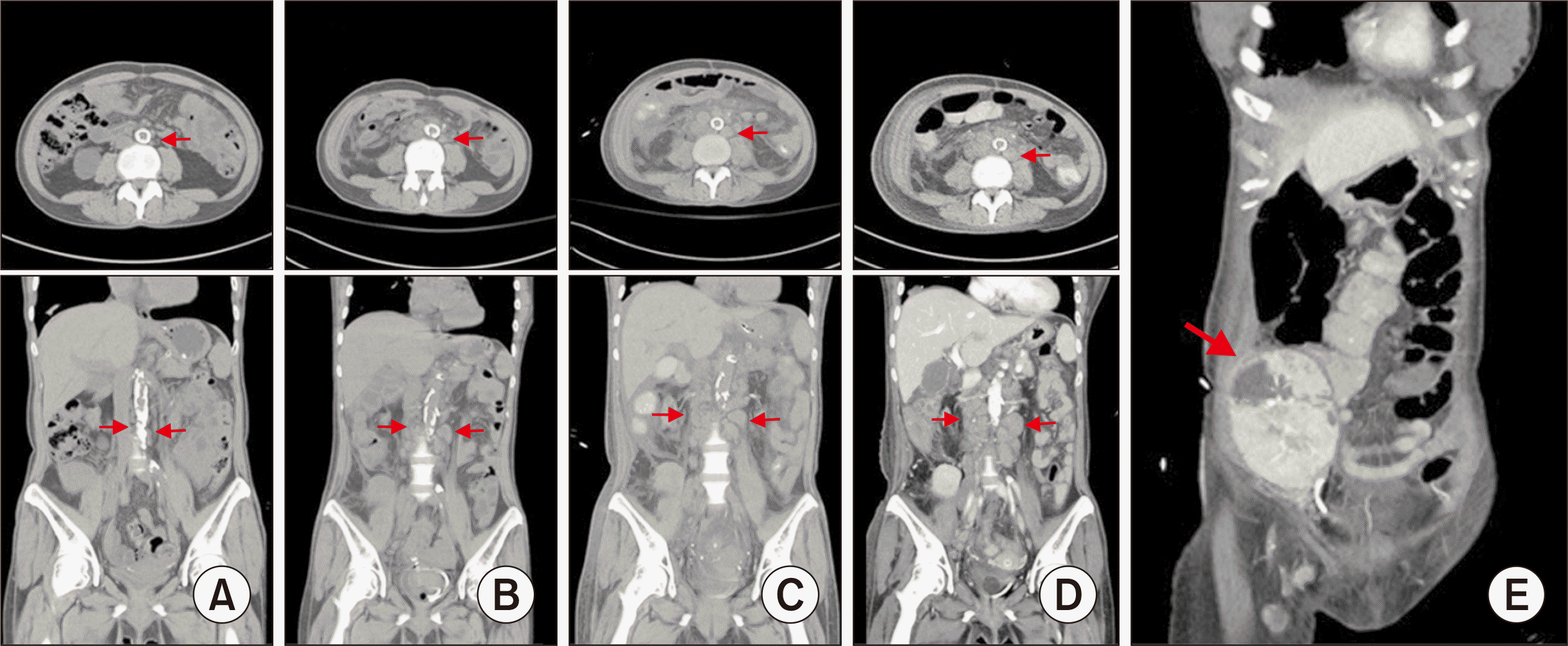 | Fig. 3Axial and coronal view of a noncontrast abdominopelvic computed tomography (CT) scan showing multiple lymphoproliferative lesions at (A) 12 weeks, (B) 17 weeks, and (C) 18 weeks after transplant. Contrast abdominopelvic CT scan showing (D) enlarged lymphoproliferative lesions and (E) necrosis in the allograft (arrows) at 19 weeks after transplant. 
|
Intraoperatively, the kidney parenchyma was found to be entirely necrotic, with lymphoproliferative lesions diffusely formed around the iliac artery. The allograft was removed and histologically examined. A gross histological examination revealed a well-demarcated, irregular, solid mass (8.6 × 5.5 × 3.6 cm) invading the renal pelvis from the renal sinus (
Fig. 4). KS was diagnosed and identified as HHV-8-positive on immunohistochemical staining (
Fig. 5). The excised lymphoproliferative lesions were also HHV-8-positive KS lesions (
Fig. 6).
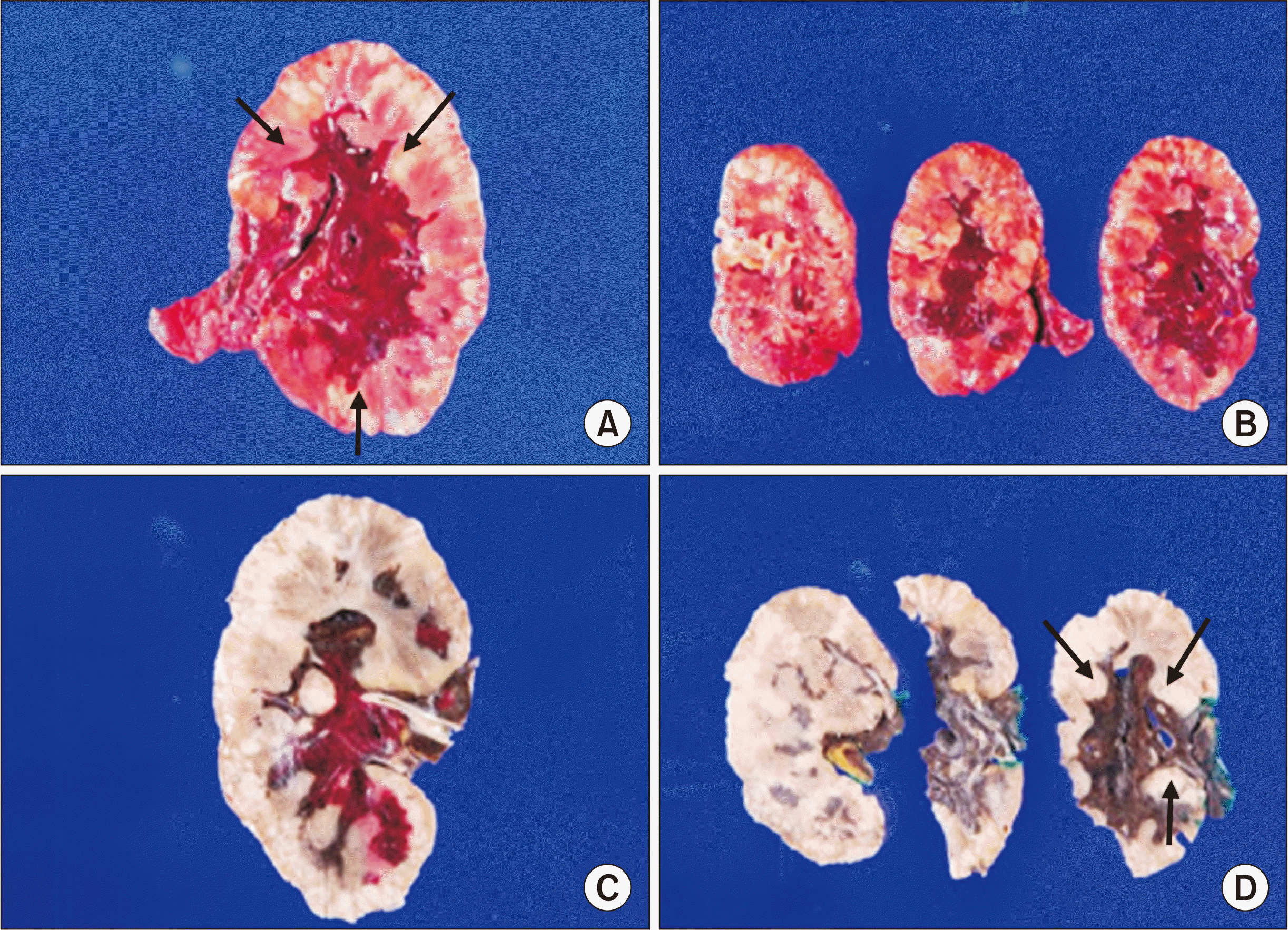 | Fig. 4Macroscopic findings of renal allograft after graftectomy. (A, B) An infiltrative hemorrhagic mass (8.6 × 5.5 × 3.6 cm; arrows) in the renal parenchyma and severe hemorrhagic findings were identified in the renal parenchyma (fresh specimen). (C, D) Involvement of the renal pelvis (arrows) from the renal sinus was identified (formalin-fixed specimen). 
|
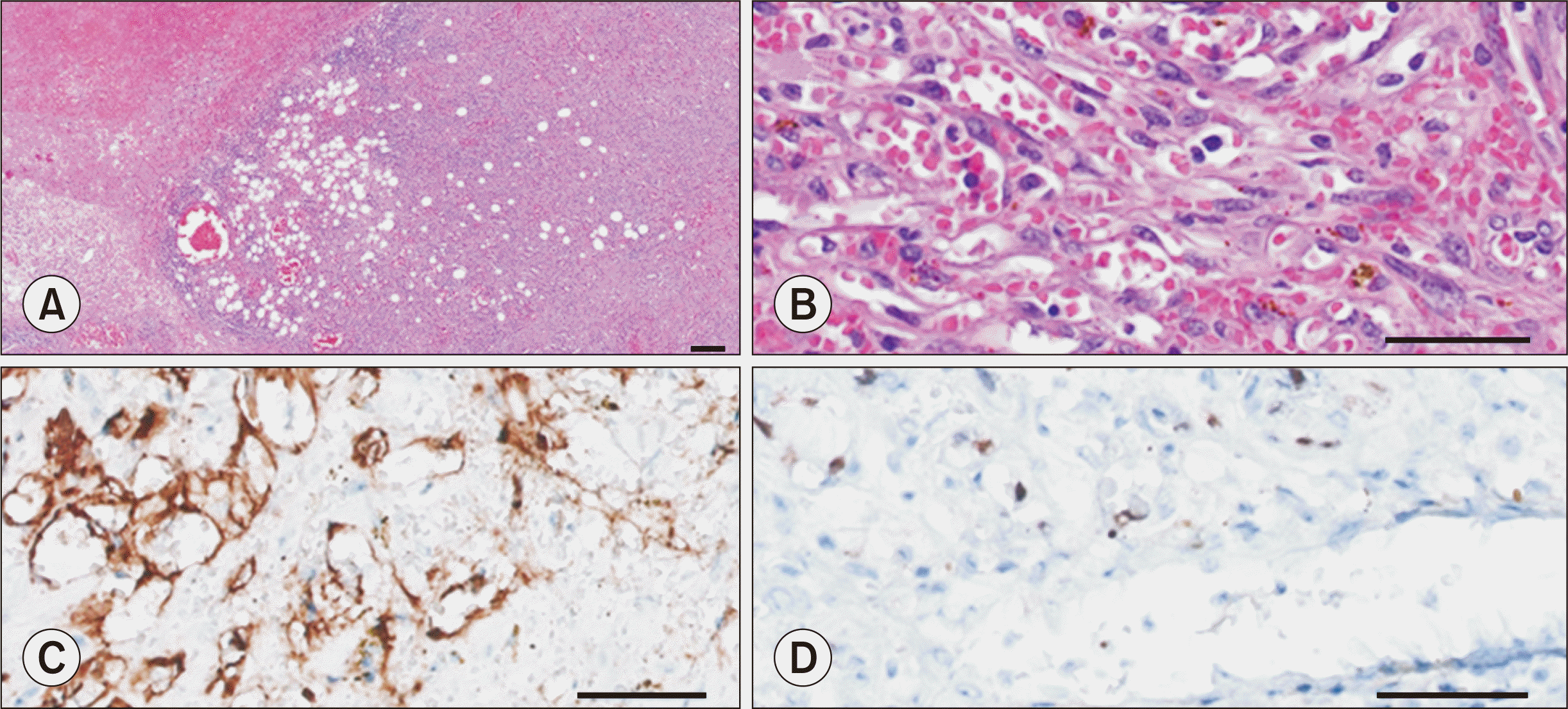 | Fig. 5Histological findings of renal allograft after graftectomy. (A) High cellular appearance in an invasive tumor nodule-like lesion (H&E, ×50). (B) Proliferation of spindle cells with a slit-like vascular space containing erythrocytes (H&E, ×400). (C) CD31 staining of spindled tumor cells (×400). (D) The spindle cells were positive for human herpesvirus 8 (immunohistochemistry human herpsevirus 8 stain, ×400). 
|
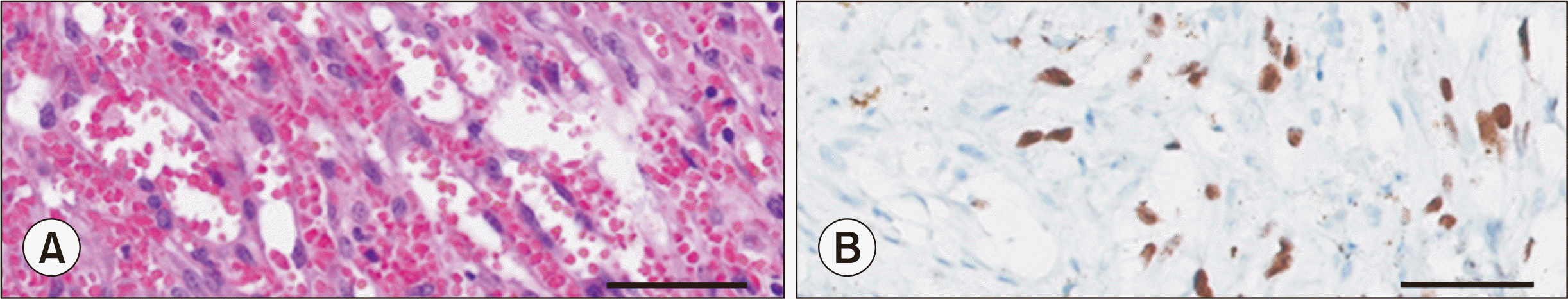 | Fig. 6Histological findings of resected lymphoproliferative lesions. (A) Cellular spindle cell proliferation with the extravasation of numerous erythrocytes (H&E, ×400). (B) The spindle cells were positive for human herpesvirus 8 (immunohistochemistry human herpsevirus 8 stain, ×400). 
|
After graftectomy, the patient’s fever and leukocytosis improved with hemodialysis without receiving any immunosuppressants or chemotherapy. She was discharged after infection control, but an abdominopelvic CT scan 3 weeks postgraftectomy showed persistent lymphadenopathy. Consequently, the patient has been scheduled for yearly follow-up observations.
Go to :

DISCUSSION
Organ transplantation [
3] not only increases the risk of infection from the use of immunosuppressive drugs, but it also increases the risk of
de novo cancer. KS is a disease that remains largely unknown among the general public; however, it has a high prevalence among organ transplant recipients [
4]. In South Korea, the expected prevalence of KS following kidney transplantation was reported to be higher than those of other posttransplantation
de novo cancers [
5].
KS can be classified into four subtypes: organ-transplant-associated, epidemic (AIDS-related), endemic (a unique aggressive subtype that occurs in equatorial African patients without HIV), and classic [
6]. It often appears in patients with organ transplants in countries like Egypt and Algeria on the Mediterranean coast of Africa. In equatorial African countries such as Uganda, Tanzania, and Congo, the prevalence of KS is about 16 per 1,000, which is higher than in other regions. This higher prevalence is related to the high HHV-8 seroprevalence in these areas [
7]. HHV-8 is associated with all forms of KS, including posttransplant KS. Viral proteins that induce KS-related cell changes disrupt the host's immune system, thereby allowing HHV-8 to survive and proliferate [
8]. The histopathological features of KS include the presence of spindle cells, which usually exhibit positive staining for endothelial cell markers, such as CD31 [
9]. Importantly, spindle cells produce cytokines that can enhance the cell proliferation of KS. HHV-8 is also a potent derivative of this cytokine, which promotes the regeneration of blood vessels. As HHV-8 DNA (deoxyribonucleic acid) is localized to tumor endothelial cells and spindle cells, HHV-8 positivity is observed in the presence of spindle cells in almost all KS lesions [
10].
The most common site for KS lesions is the skin. Even when visceral lesions are present, they usually affect the lymph nodes, lungs, and gastrointestinal tract rather than allografts [
11]. In this case, we encountered not only lymph node and lymphoproliferative lesions, but also KS invading the allograft itself [
3]. We compared our case with three previously reported cases where KS occurred in allografts [
12-
14]. Our case is unique in that it showed only an increase in creatinine levels during regular checkups without symptoms, and that the diagnosis was made in a notably shorter period of time after transplantation (
Table 1).
Table 1
Comparison of our case and previous reports on Kaposi’s sarcoma occurring in allografts
|
Detail |
Butkus et al. (1989) [12] |
Diaz-Candamio et al. (1998) [13] |
Rha et al. (2000) [14] |
Current study |
|
Age (yr) |
48 |
56 |
28 |
53 |
|
Sex |
Male |
Male |
Female |
Female |
|
Underlying disease |
MPGN |
PCKD |
Lupus nephritis |
DM ESRD |
|
Donor type |
Deceased donor |
Deceased donor |
Deceased donor |
Deceased donor |
|
Immunosuppression regimen |
Prednisolone, cyclosporine, azathioprine |
Prednisolone, cyclosporine, azathioprine |
Prednisolone, cyclosporin |
Prednisolone, tacrolimus, mycophenolate mofetil |
|
Time to diagnosis after transplantation |
8 mo |
7 mo |
10 yr |
10 wk |
|
Location of KS |
Allograft, arm, chest, groin, ankle |
Allograft, iliac vessel |
Allograft, ureter, urinary bladder, stomach, duodenum |
Allograft |
|
Initial symptom |
Surface nodular lesion in the arm/chest |
Leg edema, groin pain |
Leg edema, nausea |
None |
|
Creatinine (mg/dL) |
NA (mentioned as stable) |
1.40 |
7.64 |
2.99 |
|
Initial CT image |
No specific finding |
Infiltrating lesion involving the allograft sinus and hilum, producing mild hydronephrosis |
Multiple well-enhanced nodular masses in the urinary bladder, ureter, and renal pelvis of the transplanted kidney with hydronephrosis |
Mild swelling of the transplanted kidney with perinephric infiltration |
|
Treatment |
Immunosuppression withdrawal, graftectomy |
Immunosuppression withdrawal, chemotherapy |
Immunosuppression withdrawal, chemotherapy |
Graftectomy |

In general, regression of KS lesions can be achieved by reducing/discontinuing immunosuppressants or by using mTOR inhibitors; in cases of disseminated or visceral KS that do not respond to these treatments, combination chemotherapy may be initiated [
15]. However, as in this case, it was unclear whether the KS generated in the allograft itself would be amenable to such treatments. We could not explain the decrease in renal function or the abnormal blood flow of the transplanted kidney, and KS could not be diagnosed until graftectomy and histological examination confirmed it. Therefore, we did not attempt to discontinue immunosuppressants or use mTOR inhibitors. There was also no consideration of chemotherapy when the effect was minimal.
This case demonstrates that allografts can be a site of KS lesions within a short period of time after transplantation. In the context of transplantation, without rejection or infection, if there is an unexplained decrease in renal function or if necrosis or blood flow disorders of transplanted kidneys are observed after kidney transplantation, it is advisable to consider the possibility of de novo cancer, such as KS invading the allograft itself.
Go to :








 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download