Abstract
Laparoscopic donor nephrectomy (LDN) is increasingly popular because of its advantages over open surgery. Chyle leak after donor nephrectomy is a rare but potentially lethal complication if not treated appropriately. We describe a case of a 43-year-old female patient with no remarkable history who presented a chyle leak on day 2 after right transperitoneal LDN. Since conservative treatment failed, the patient underwent magnetic resonance imaging (MRI) and intranodal lipiodol lymphangiography, which confirmed the chyle leak from the right lumbar lymph trunk into the right renal fossa. The chyle leak was percutaneously embolized twice, on postoperative day (POD) 5 and POD 10, by a mixture of N-butyl-2-cyanoacrylate and lipiodol. The drainage fluid decreased significantly after the second embolization. The subhepatic drainage tube was withdrawn on POD 14, and the patient was discharged on POD 17. MRI lymphangiography and intranodal lipiodol lymphangiography effectively identified the chyle leak point. Percutaneous embolization seems to be a safe, effective method for treating high-output chyle leaks.
Laparoscopic donor nephrectomy (LDN) is increasingly popular because of its advantages over open surgery [1-3]. Chyle leak after LDN is a rare but potentially lethal complication if not treated appropriately [4-7]. To date, no guideline exists for treating chyle leak [4,5,7,8]. Most reported cases were successfully treated with conservative therapy, and only a few required radiographic or surgical intervention [4-7,9-11]. We describe a case of a chyle leak after right transperitoneal LDN, in which conservative treatment failed. The patient was then successfully treated by percutaneous embolization. This paper highlights a rare complication of right LDN and percutaneous embolization therapy.
This study was approved by the Institutional Review Board of Viet Duc University Hospital (IRB No. 01.2023.NCVD). Consent for publication was obtained for individual person’s data included in the study during the post-withdrawal visit, noted in the patient's paper medical record.
A 43-year-old female patient with no remarkable history was admitted to our institution for a kidney donation to her relative. The patient underwent right transperitoneal LDN. On postoperative day (POD) 2, the subhepatic drainage was over 1,500 mL per day of milky fluid. Biochemical testing of the fluid detected a triglyceride level of 20.12 mmol/L and a cholesterol level of 2.21 mmol/L, confirming a chyle leak. Initially, the patient was treated with fasting and total parenteral nutrition. Three days later, the drainage fluid slightly decreased to 1,100 mL per day. Therefore, magnetic resonance imaging (MRI) lymphangiography was performed, revealing extravasation of gadolinium into the right renal fossa from the right lumbar lymph trunk (LLT) at the level of the L2 vertebra. The thoracic duct drained from the left lumbar trunk, and the cisterna chyli was not seen (Fig. 1). Next, the patient underwent lipiodol lymphangiography. On lymphangiography, lipiodol extravasation was noted from the upper end of the right LLT into the right renal fossa, confirming the chyle leak (Fig. 2A). The left LLT had a normal appearance and connected directly to the thoracic duct without visible cisterna chyli (Fig. 2A). We immediately punctured the right LLT, using a Chiba 22G needle, and blocked it by a 3-mL mixture of N-butyl-2-cyanoacrylate (NBCA) and lipiodol (1:3.5 ratio) under fluoroscopy (Fig. 2B). After embolization, the patient continued parenteral nutrition for 5 days.
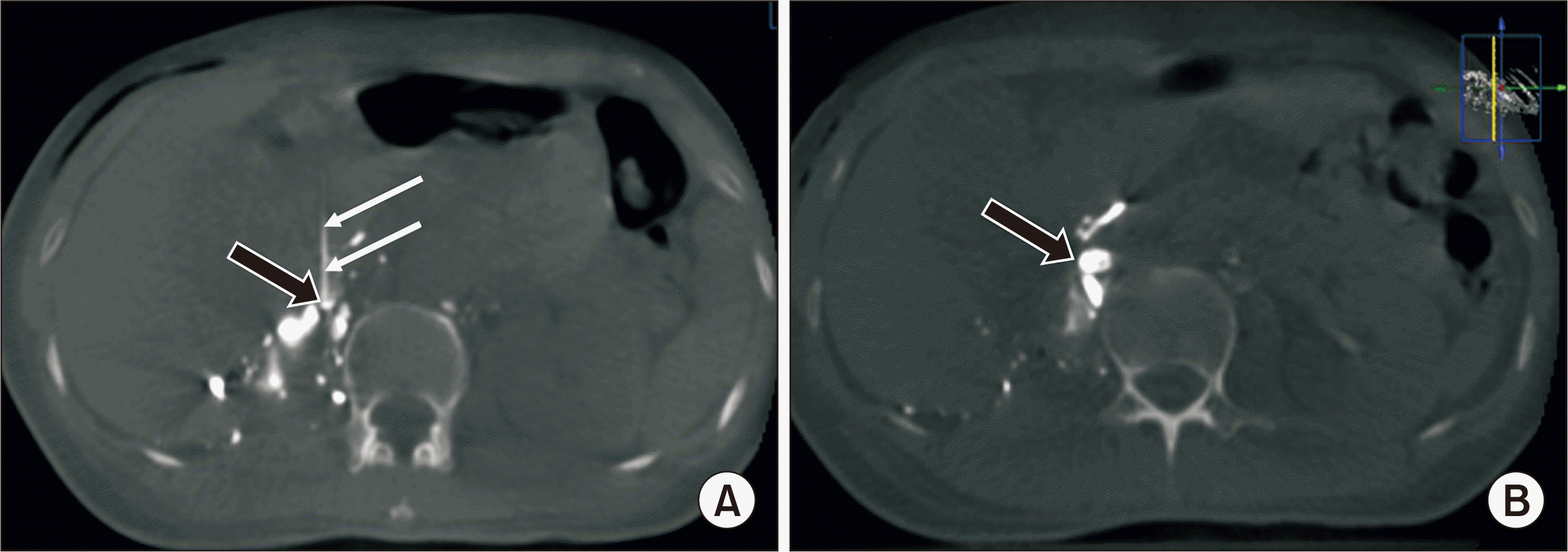
The drainage fluid decreased but remained at 500 mL per day. Therefore, we decided to perform a second percutaneous embolization on POD 10. Fluoroscopy before the procedure showed that most of the previous mixture of NBCA and lipiodol (1:3.5 ratio) was washed into the right renal fossa (Fig. 3A). Then, after accessing the right LLT with a 22-G Chiba needle, an XperCT (Philips) examination on the table was performed to confirm the needle tip's position in the right LLT (Fig. 4A). Next, a mixture of NBCA and lipiodol (1:2 ratio) was injected directly through a Chiba 22G needle to occlude the right LLT (Fig. 3B). After that, an XperCT examination was also done to confirm the concentration of NBCA in the right LLT (Fig. 4B). The next day, the drainage decreased significantly and became dry before being removed on POD 14. The patient returned to a regular diet on POD 14 and was discharged on POD 17. A postoperative examination at 1 month showed that the patient was stable, and there was no fluid accumulation in the peritoneum or the right renal fossa.
In typical anatomy, the cisterna chyli is a dilated lymphatic sac located in the retroperitoneal space, behind the right crus of the diaphragm (the right retrocrural space), and immediately right to the aorta [12]. It is formed by the junction of the two LLTs and the intestinal lymphatic trunk at the level of L1–2 [12]. The cisterna chyli receives and drains lymphatic fluid from the lower extremities, the abdomen, and the pelvis to the thoracic duct. However, anatomical variations of the cisterna chyli and thoracic duct are quite common. In up to 50% of cases, the cisterna chyli can be absent and is replaced by the lymphatic plexus [12]. In our patient, the cisterna chyli was not observed. Two LLTs ran parallel on either side of the L1–2 vertebrae, potentially replacing the cisterna chyli. This variant may lead to damage to the right LLT during LDN.
Chyle leak after LDN is a rare complication [5,6,9,10]. Because of its location immediately right to the abdominal aorta, the cisterna chyli and its branches can be damaged during dissection of the kidney and renal vessels. Attempts to ligate the renal artery proximal to its origin further increase the risk of lymphatic injury. For this reason, lymphatic fistula complications are more common after right donor nephrectomy than after left nephrectomy. However, most reports of lymphatic leakage after donor nephrectomy occurred on the left instead of the right side [4-6,9,10,13]. An explanation for this could be that donor nephrectomy is likely to be performed more often on the left side than on the right side. At our institution, right donor nephrectomy is performed routinely [14] and is similar to the corresponding procedure on the left side. Furthermore, laparoscopy seems to cause a higher risk of lymphatic fistula than laparotomy [15]. Several reasons can explain this: first, with open surgery, the surgeon can easily manipulate suturing and clipping, have better control in the surgical field, and can immediately handle injuries of lymphatic vessels. Second, laparoscopic surgery with high CO2 pressure can hide lymphatic injuries. Some authors recommend systematically coagulating the lymphatic channels adequately to avoid possible lymphatic fistula.
Clinically, chylous fistula manifests in many forms: asymptomatic or apparent chylous ascites, retroperitoneal fluid collection, and chylothorax. The symptoms are more severe when lesions are larger and when they are detected later. Once it occurs, a chylous fistula can be easily recognized by its characteristic milky color. Laboratory tests help confirm the diagnosis; if the fluid has a triglyceride level >1.24 mmol/L (200 mg/dL), with a cholesterol level <5.18 mmol/L, chylomicrons are present [8]. Imaging modalities such as ultrasonography and X-ray examinations cannot distinguish lymphatic leak from other causes, but allow an assessment of severity and provide guidance for an intervention if necessary. MRI and intranodal lipiodol lymphangiography enable precisely identifying the leak's site to plan the surgery or intervention.
Although there is no consensus on treatment for chyle leaks, most authors recommend starting with conservative treatment. Dietary control (low fat, high protein, medium-chain triglyceride diet) or fasting and total parenteral nutrition could be applied to minimize the volume of chyle. Paracentesis is done to relieve symptoms depending on the development of chyle leakage. If conservative treatment is ineffective, a percutaneous intervention is mainly indicated instead of surgical repair due to its benefits. A mixture of NBCA and lipiodol (at a ratio of 1:2 to 1:4) with or without metallic coils is usually used to occlude the leak point. Embolization under fluoroscopy is most commonly performed because it provides real-time X-ray imaging. Kwon et al. [13] reported six cases of chylous ascites after retroperitoneal surgery, including four left kidney donors. In all patients, residual chyle leaks after medical and surgical treatment were successfully treated by lymphopseudoaneurysm NBCA embolization without serious complications. Some authors used computed tomography (CT) instead of fluoroscopy to guide the chyle leak embolization [16]. CT guidance helps to precisely access the leak point and avoid injuring adjacent blood vessels, but a drawback of this technique is the inability to perform real-time imaging during the injection. In our patient, the chyle leak persisted after the first embolization, although it minimally decreased. Most of the previous mixture of NBCA and lipiodol (1:3.5 ratio) was washed into the right renal fossa on fluoroscopy before the second embolization. Therefore, in the second embolization, we used a thicker mixture (1:2 ratio) for better embolization. XperCT imaging on the table was also performed before and after embolization to ensure effective target occlusion.
Chyle leak is a rare complication after laparoscopic kidney donation surgery. Anatomical variation of the lymphatic system may be associated with the postoperative complication of chyle leak. MRI lymphangiography and intranodal lymphangiography are valuable for accurately diagnosing the leak point. Percutaneous embolization is minimally invasive and can be effective in cases where conservative therapy fails.
ACKNOWLEDGMENTS
Author Contributions
Conceptualization: all authors. Data curation: TVS. Formal analysis: LTD, THP. Methodology: LNV. Project administration: all authors. Visualization: NQN, LTD. Writing–original draft: TVS, LNV. Writing–review & editing: all authors. All authors read and approved the final manuscript.
REFERENCES
1. Shafizadeh S, McEvoy JR, Murray C, Baillie GM, Ashcraft E, Sill T, et al. 2000; Laparoscopic donor nephrectomy: impact on an established renal transplant program. Am Surg. 66:1132–5. DOI: 10.1177/000313480006601208. PMID: 11149584.

2. Simon SD, Castle EP, Ferrigni RG, Lamm DL, Swanson SK, Novicki DE, et al. 2004; Complications of laparoscopic nephrectomy: the Mayo Clinic experience. J Urol. 171:1447–50. DOI: 10.1097/01.ju.0000117942.61971.41. PMID: 15017195.

3. Minnee RC, Idu MM. 2010; Laparoscopic donor nephrectomy. Neth J Med. 68:199–206.
4. Seth A, Sharma A, Kenwar DB, Singh S. 2019; Chylous ascites: complication of laparoscopic donor nephrectomy. Case report and review of literature. Transplantation. 103:e74–8. DOI: 10.1097/TP.0000000000002514. PMID: 30399121.

5. Capocasale E, Iaria M, Vistoli F, Signori S, Mazzoni MP, Dalla Valle R, et al. 2012; Incidence, diagnosis, and treatment of chylous leakage after laparoscopic live donor nephrectomy. Transplantation. 93:82–6. DOI: 10.1097/TP.0b013e31823b2d8e. PMID: 22143459.

6. Bachmann A, Ruszat R, Dickenmann M, Giannini O, Mayr M, Steiger J, et al. 2005; Chyloretroperitoneum with secondary chylothorax after retroperitoneoscopic donor nephrectomy. Urology. 66:881. DOI: 10.1016/j.urology.2005.04.025. PMID: 16230168.

7. Leibovitch I, Mor Y, Golomb J, Ramon J. 2002; The diagnosis and management of postoperative chylous ascites. J Urol. 167(2 Pt 1):449–57. DOI: 10.1016/S0022-5347(01)69064-5. PMID: 11792897.

8. Lv S, Wang Q, Zhao W, Han L, Wang Q, Batchu N, et al. 2017; A review of the postoperative lymphatic leakage. Oncotarget. 8:69062–75. DOI: 10.18632/oncotarget.17297. PMID: 28978181. PMCID: PMC5620321.

9. Simon SP, Thomas J, Nair TB, Bhat SH. 2020; A rare complication after laparoscopic donor nephrectomy: chyle leak-a case report. Indian J Transplant. 14:374–6. DOI: 10.4103/ijot.ijot_43_20.

10. Kalia S, Narkhede A, Yadav AK, Bhalla AK, Gupta A. 2022; Retrograde transvenous selective lymphatic duct embolization in post donor nephrectomy chylous ascites. CEN Case Rep. 11:1–5. DOI: 10.1007/s13730-021-00618-6. PMID: 34218419. PMCID: PMC8811106.

11. Jairath A, Singh A, Ganpule A, Mishra S, Sabnis R, Desai M. 2015; Management protocol for chylous ascites after laparoscopic nephrectomy. Urology. 86:521–8. DOI: 10.1016/j.urology.2015.06.001. PMID: 26210005.

12. Pinto PS, Sirlin CB, Andrade-Barreto OA, Brown MA, Mindelzun RE, Mattrey RF. 2004; Cisterna chyli at routine abdominal MR imaging: a normal anatomic structure in the retrocrural space. Radiographics. 24:809–17. DOI: 10.1148/rg.243035086. PMID: 15143230.

13. Kwon LM, Hur S, Jeong CW, Jae HJ, Chung JW. 2021; Glue embolization of lymphopseudoaneurysm for chylous ascites after retroperitoneal surgery. Korean J Radiol. 22:376–83. DOI: 10.3348/kjr.2020.0056. PMID: 32901460. PMCID: PMC7909856.

14. Vu LN, Nghia NQ, Thanh DT, Giang TB, Nga VT, Bui LM, et al. 2019; Laparoscopic living donor right nephrectomy: assessment of outcome and association of BMI to length of right renal vein. Actas Urol Esp (Engl Ed). 43:536–42. DOI: 10.1016/j.acuroe.2019.05.009.

15. Tiong HY, Goel RK, White WM, Goldfarb DA, Kaouk JH. 2015; Chylous ascites after laparoscopic donor nephrectomy. Asian J Endosc Surg. 8:34–9. DOI: 10.1111/ases.12144. PMID: 25384614.

16. Itou C, Koizumi J, Myojin K, Yamashita T, Mori N, Imai Y. 2013; A case of refractory chylous ascites after nephrectomy successfully treated with percutaneous obliteration using adhesive glue. Jpn J Radiol. 31:71–4. DOI: 10.1007/s11604-012-0146-8. PMID: 23065489.

Fig. 1
Magnetic resonance imaging (MRI) lymphangiography. (A, B) T1 Viber fat-sat in the axial images and (C) coronal maximum intensity projection (MIP) image showing extravasation of contrast from the right lumbar lymph trunk to the right renal fossa (black arrows). The cisterna chyli was not seen. Right and left lumbar lymph trunks (white arrows).
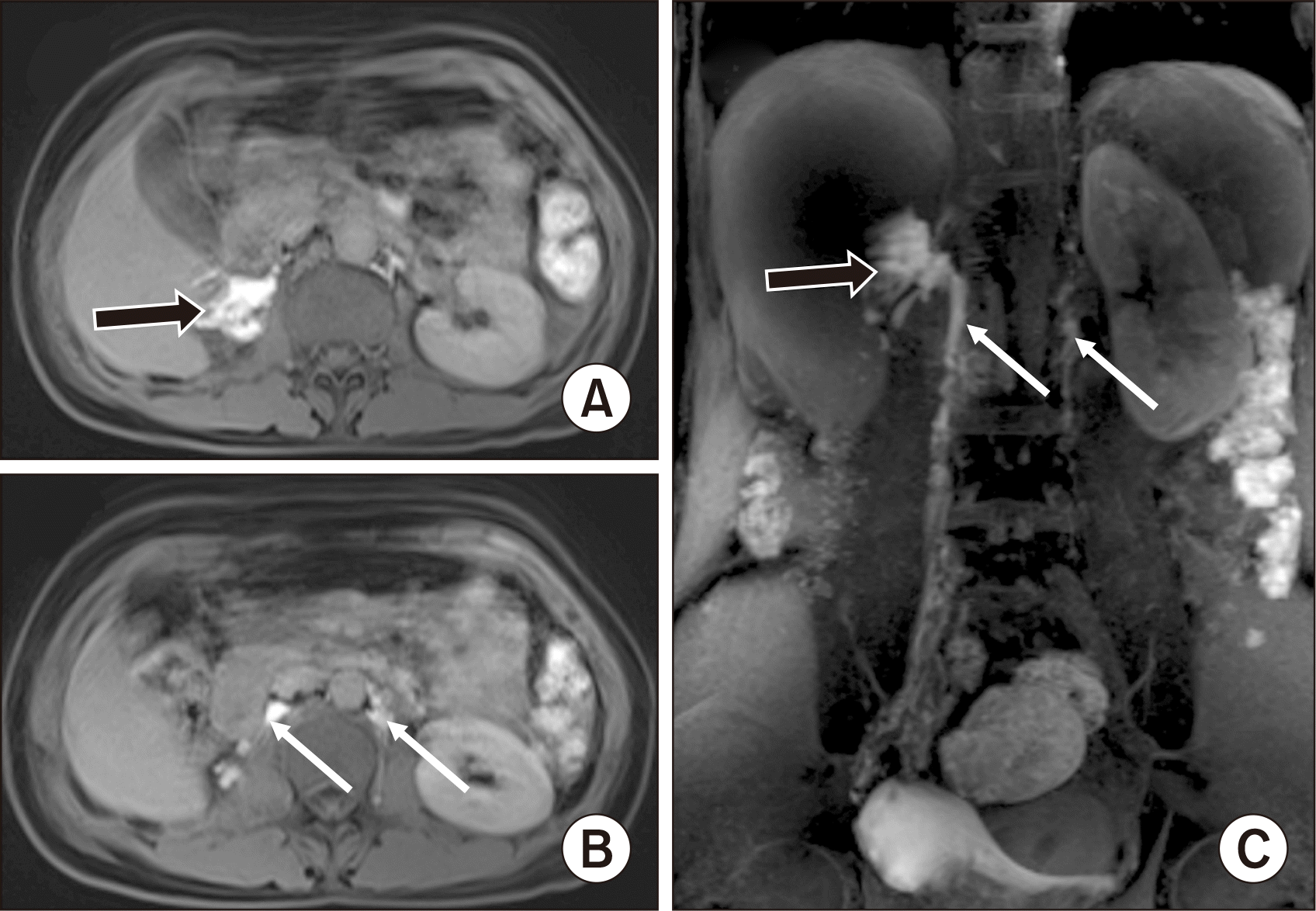
Fig. 2
(A) Intranodal lymphangiography on postoperative day 5, confirming the extravasation of lipiodol from the right lumbar lymph trunk (black arrow) to the right renal fossa (asterisk). White arrows: thoracic duct. Arrowheads: subhepatic drainage. (B) Single-shot image after the first embolization, showing glue in the right lumbar lymph trunk (arrowheads) and right renal fossa (asterisk).
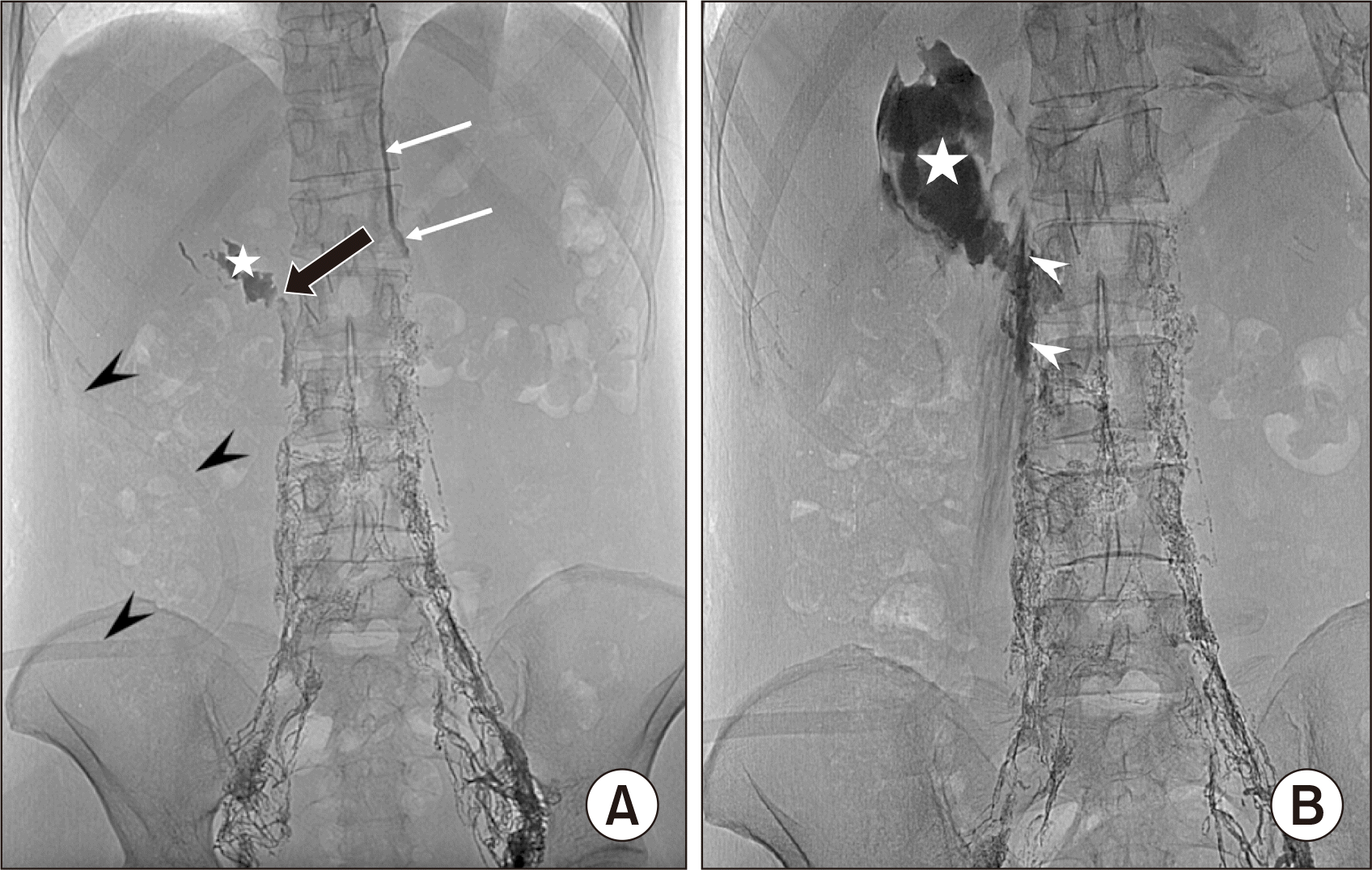
Fig. 3
(A) Fluoroscopy on postoperative day 10 showing that most of the mixture of N-butyl-2-cyanoacrylate (NBCA) and lipiodol was washed from the right lumbar trunk (black arrows) into the right renal fossa (asterisk). (B) Single-shot image after the second embolization: the right lymph trunk (arrowheads) was completely embolized by a mixture of NBCA and lipiodol (1:2 ratio) through a 22G Chiba needle (white arrows). Asterisk: lipiodol in the right renal fossa.
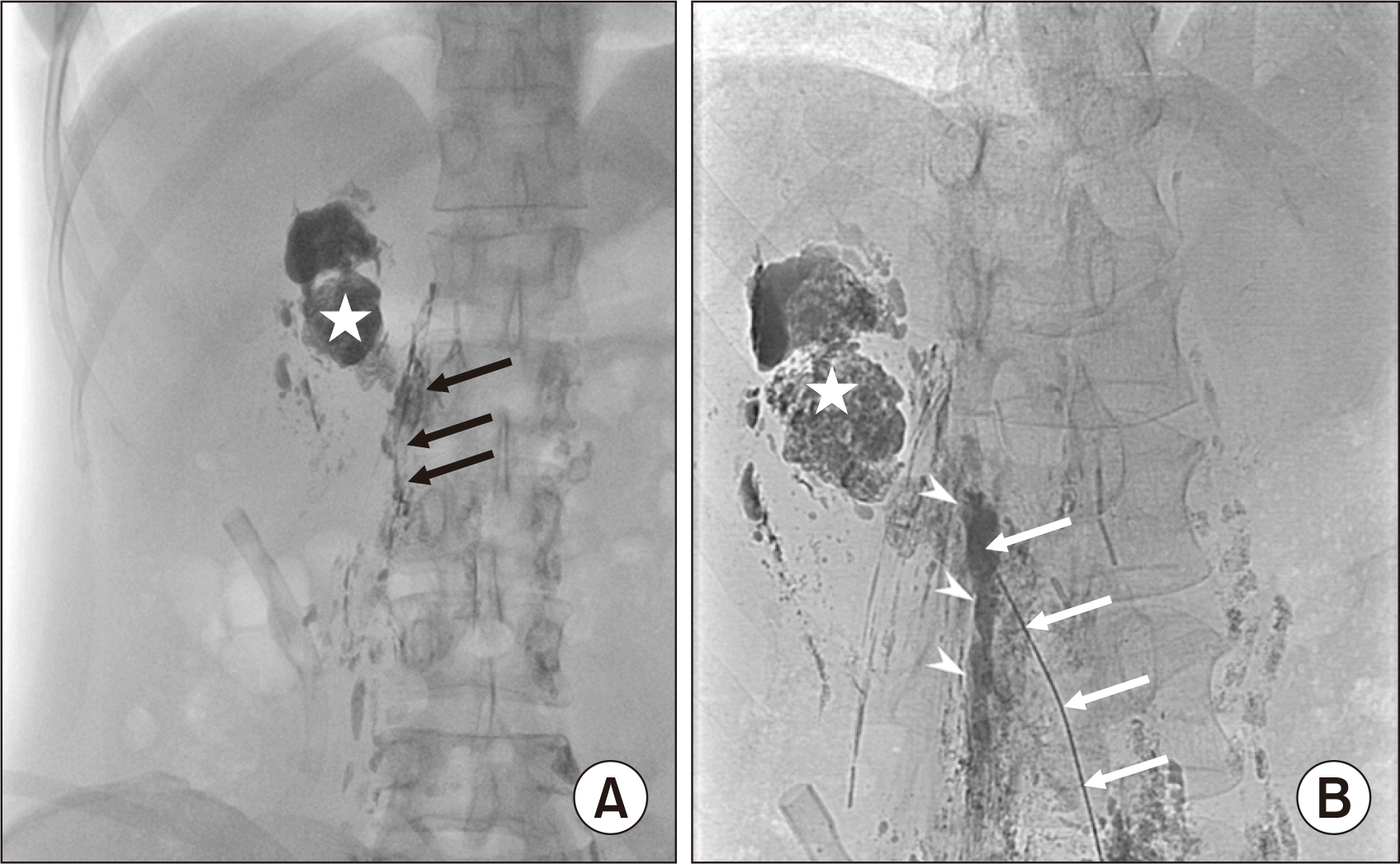
Fig. 4
XperCT (Philips) on the table before (A) and after (B) glue injection in the second embolization. (A) The right lumbar lymph trunk (black arrow) was accurately accessed by a Chiba needle (22G; white arrows). (B) After the second embolization, the right lumbar lymph trunk (arrow) was completely embolized.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download