Abstract
The adoption of high-sensitivity flow cytometry (HSFC) in routine laboratory settings has been slow owing to concerns regarding the reliability and reproducibility of results. Validation is an essential prerequisite for conducting assays, and implementing the CLSI guidelines has been confusing, primarily because many aspects are not yet established. We aimed to validate an HSFC protocol for detecting follicular helper T (Tfh) cells in a real-world laboratory environment. The analytical validity of the Tfh cell panel was ensured through rigorous testing, including evaluations of precision, stability, carryover, and sensitivity, following the CLSI H62 guidelines. We found that Tfh cells, present in very small numbers in the blood, could be sufficiently detected through HSFC, and concerns about the reliability and reproducibility of the results in real-world laboratories could be solved through systematic validation. Establishing the lower limit of quantification (LLOQ) is a critical step in HSFC evaluations. By selecting an appropriate sample, for example, collecting residual cells from CD4 isolation in our experiment and using them as low-level samples, the LLOQ could be accurately established. The strategic validation of flow cytometry panels can facilitate the adoption of HSFC in clinical laboratories, even with limited resources.
High-sensitivity flow cytometry (HSFC) has become a valuable tool in biomedical research because it can detect rare events and quantify small changes in cellular populations. HSFC can measure cell populations comprising less than 0.1% of the total cell population [1]. Despite its advantages, adopting HSFC in routine laboratory settings has been slow, partly because of concerns regarding the reliability and reproducibility of the results. However, HSFC is increasingly being used [2], underscoring the need for standardized validation methods. Given that laboratory-developed assays are commonly used for flow cytometry, the CLSI H62 announcement appears to be consistent with the recent regulatory changes to these assays. Although validation is an essential prerequisite for conducting the test, implementing the CLSI guidelines in clinical laboratories has been confusing, primarily because many aspects are not yet established. Furthermore, given the restricted personnel and resources in clinical laboratories, practical guidelines for essential items are necessary to meet all conditions suggested by the CLSI guidelines. We report our experience validating an HSFC protocol for detecting follicular helper T (Tfh) cells in a real-world laboratory environment.
Tfh cells, a distinct subset of helper T cells, are important for immune modulation and cancer development and function by regulating germinal center B cell differentiation signals [3]. The percentage of Tfh cells in the blood varies depending on different factors, such as age, health status, and the immune response [3-6]. In healthy adults, Tfh cells typically comprise approximately 1%–3% of circulating CD4+ T cells [7]. The Tfh panels were validated against the minimum categories required in real-world laboratory settings. The analytical validity of the Tfh panel was ensured through rigorous testing, including evaluations of precision, stability, carryover, and sensitivity measures, following the CLSI H62 guidelines and previous literature [8-10]. The study was conducted from December 7, 2021, to January 20, 2022. To exclude matrix effects, validation studies were conducted using residual peripheral blood (PB) samples (N=17) obtained after performing a complete blood cell count at Asan Medical Center (Seoul, Korea). Given the rarity and instability of Tfh cells in PB samples, we stabilized and fixed these cells by admixing each PB sample with TransFix (Cytomark Limited, Buckingham, UK). To increase the proportion of Tfh cells in the test samples, CD4+ T cells were isolated using the EasySep Isolation Kit (STEMCELL Technologies, Inc., Vancouver, Canada) before the assay. Our choice of multicolor reagent combinations for analyzing Tfh cells and their subtypes was based on previous studies [11, 12] and included the following antibodies (all from BD Biosciences, San Jose, CA, USA): anti-CD45-RA BV510 with viability aqua dye, anti-CD3-APC-H7, anti-CD4-BV421, anti-CXCR5-PerCP-Cy5.5, anti-CCR6-PE, anti-CXCR3-FITC, anti-PD-1-PE-CF594, and anti-ICOS-Alexa Fluor 647. Tfh cells were defined as CD3+/CD4+/CXCR5+ cells, and Tfh subtypes were classified based on their CCR6 and CXCR3 expression patterns: type 1 follicular helper T cells (Tfh1; CXCR3+/CCR6−), type 2 follicular helper T cell (Tfh2; CXCR3−/CCR6−), and Tfh17 (CXCR3−/CCR6+). The primary focus of the validation studies was Tfh cells rather than their subtypes. Prior to each analytical run, isotype controls were prepared and stained with antibodies against CD45-RA BV510, CD3-APC-H7, CD4-BV421, CXCR5 IgG-PerCP-Cy5.5, CCR6 IgG-PE, and CXCR3 IgG-FITC. Tfh cell counts were obtained using a Navios flow cytometer (Beckman Coulter, Brea, CA, USA). The minimum count of total cells was set to 105, with a desired CV of 10% for the frequency of Tfh cells [10]. The Tfh populations were manually gated by a laboratory technician and medical supervisor according to a predefined gating strategy (Fig. 1), and the results were compared for consistency. The study protocol was approved by the Institutional Review Board of Asan Medical Center (approval No. 2021-0591, 2022-1276).
Precision was evaluated using two approaches: intra- and inter-assay precision. Intra-assay precision was determined by measuring three replicates of each sample in a single run, whereas inter-assay precision was determined by measuring three replicates of each sample in four different runs. Our Tfh assay was designed for research purposes and can be validated with a minimum of three samples [10]. However, for patient testing, a minimum of six samples is necessary for precision validation owing to the expected higher variability in low-level samples [1]. Intra-assay precision was expressed as the %CV for reportable results for each sample, whereas inter-assay precision was expressed as the mean %CV of all runs for each sample. Regarding the acceptable precision criterion, the CV should be within 10%, with a CV range of up to 25% considered acceptable. However, for detecting rare cell events, a CV range of 30%–35% can be considered acceptable according to CLSI H62 [12]. Therefore, we adopted an acceptable precision range of a 30%–35% CV. Regarding intra-assay precision, all %CVs for Tfh cells and their subtypes were <10%. Although some %CVs for the Tfh subtypes were higher than 10%, most Tfh results exhibited high inter-assay precision (Table 1).
Sample stability was assessed to evaluate the effect of TransFix (Cytomark Ltd.) mixing and sample time lapses. One patient’s sample was divided into two groups: one was mixed with TransFix (Cytomark Ltd.), and the other was left untreated. Both were prepared based on CD4 isolation. The samples were stored at room temperature (20°C–22°C) and analyzed at five time points (0, 4, 24, 28, and 48 hours) after preparation. The fraction of total lymphocytes, including Tfh cells and their subtypes, was calculated at each time point. The relative difference between the results obtained at each time point and the baseline results obtained immediately after cell processing was calculated. A relative difference <20% indicated sample stability. Analysis of untreated sample stability revealed significant variations in the relative differences in Tfh subtypes after 4 hours. However, Tfh cells remained stable for up to 48 hours, irrespective of whether TransFix was mixed (Supplemental Data Table S1). The practice of testing immediately after sample collection can reduce stability issues, as is performed in our laboratory. However, in case of delays, stability should be confirmed for at least three samples, including assessing changes in markers or cellular subsets during storage/shipment, to account for potential loss or alterations.
For validated carryover, high-level Tfh samples (Sample A) were obtained from patients with autoimmune conditions, whereas low-level Tfh samples (Sample B) were obtained from healthy individuals who did not undergo CXCR5 staining during a medical examination. The samples were alternated and analyzed three times with the following sequences: A1, B1, A2, B2, A3, and B3. The carryover was calculated using Equation 1:
Carryover (%)=([B1−B3])/([A3−B3])×100 (1)
The measured Tfh values for each sample were consistent across replicates (Supplemental Data Table S2). Carryover was evaluated using Equation (1) and determined to be −0.017%; no carryover was observed.
The assay sensitivity was assessed by evaluating several key parameters, including the limit of blank (LOB), limit of detection (LOD), lower limit of quantification (LLOQ), and linearity. The LOB and LOD were validated based on the results obtained using the isotype control. Since the isotype control was not stained with CXCR5, it could have served as a Fluorescence Minus One control. Ten results from all validation runs were analyzed. The LOB was calculated as mean plus 1.645 times the SD, whereas the LOD was calculated as the LOB plus 1.645 times the SDlow-level sample. Detecting low levels of Tfh cells can be challenging because even healthy individuals can exhibit varying levels of Tfh cells depending on their physiological state [12, 13]. To address this, we used the residual cells following CD4 isolation, which were depleted of Tfh cells, as the low-level samples. The CD4-isolated samples were mixed with low-level samples and serially diluted two-fold to create five levels for each of the three samples. Each level was analyzed in triplicate, and the mean, SD, and %CV were calculated for replicates with values above the LOD. The LLOQ was calculated as the mean of the lowest level. The LLOQ data that best represented the assay performance were used to assess linearity. As the semi-quantitative method used in this study was not amenable to formal linearity verification, linearity was evaluated using R2 calculations after linear regression. The LOB was calculated to be 0.03%, and the LOD was 0.05%. The LLOQ was identified as the relative percentage of Tfh cells (0.11%) (Supplemental Data Table S3). The R2 value of the linearity was 0.9970, indicating very good linearity (Fig. 2).
This study confirms the ability of HSFC to detect Tfh cells in blood samples, even when present in very small numbers. Our study has some limitations. As we aimed to propose a HSFC validation plan that is feasible in the real world under limited laboratory resources, we conducted a limited number of tests. The systematic validation approach, which involves evaluating essential verification items with a minimum number of samples, can effectively address concerns related to the reliability and reproducibility of results in real-world laboratory settings with limited resources. Establishing the LLOQ is a crucial step in evaluating HSFC, and it can be accurately and precisely determined by selecting an appropriate sample, such as collecting residual cells from CD4 isolation and using them as low-level samples in our experiment. In summary, implementing a strategic approach to validate flow cytometry panels can accelerate HSFC adoption in clinical laboratories, even with limited resources.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; grant No. 2020R1A5A1018052) and by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (grant No. 2021IL0012).
Notes
AUTHOR CONTRIBUTIONS
Kim H contributed to the conceptualization, data analysis, data interpretation, and manuscript drafting and editing. Shin S contributed to the project administration. Kim M, Cho Y, and Jang S reviewed and edited the manuscript. Hwang S contributed to the study conceptualization, data acquisition and interpretation, and project administration and critically revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of this work.
REFERENCES
1. Sommer U, Eck S, Marszalek L, Stewart JJ, Bradford J, McCloskey TW, et al. High‐sensitivity flow cytometric assays: considerations for design control and analytical validation for identification of rare events. Cytometry Part B: Clinical Cytometry. 2021; 100:42–51. DOI: 10.1002/cyto.b.21949. PMID: 32940947.

2. Kim HY, Yoo IY, Lim DJ, Kim HJ, Kim SH, Yoon SE, et al. 2022; Clinical utility of next-generation flow-based minimal residual disease assessment in patients with multiple myeloma. Annals of Laboratory Medicine. 42:558–65. DOI: 10.3343/alm.2022.42.5.558. PMID: 35470273. PMCID: PMC9057816.

3. Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. T Follicular Helper Cells: Methods and Protocols. 2015; 199–207. DOI: 10.1007/978-1-4939-2498-1_17. PMID: 25836313.

4. Edner NM, Heuts F, Thomas N, Wang CJ, Petersone L, Kenefeck R, et al. 2020; Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nature Immunology. 21:1244–55. DOI: 10.1038/s41590-020-0744-z. PMID: 32747817. PMCID: PMC7610476.

5. Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, et al. 2014; Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathogens. 10:e1003853. DOI: 10.1371/journal.ppat.1003853. PMID: 24497824. PMCID: PMC3911819.

6. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. 2016; Follicular helper T cells. Annual Review of Immunology. 34:335–68. DOI: 10.1146/annurev-immunol-041015-055605. PMID: 26907215.

7. Danger R, Chesneau M, Delbos F, Le Bot S, Kerleau C, Chenouard A, et al. 2019; CXCR5+ PD1+ ICOS+ circulating T follicular helpers are associated with de novo donor-specific antibodies after renal transplantation. Frontiers in Immunology. 10:2071. DOI: 10.3389/fimmu.2019.02071. PMID: 31552030. PMCID: PMC6746839.
8. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. 2011; Human blood CXCR5+ CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–21. DOI: 10.1016/j.immuni.2010.12.012. PMID: 21215658. PMCID: PMC3046815.

9. Degandt S, Peeters B, Jughmans S, Boeckx N, Bossuyt X. 2018; Analytical performance of an automated volumetric flow cytometer for quantitation of T, B and natural killer lymphocytes. Clinical Chemistry and Laboratory Medicine (CCLM). 56:1277–88. DOI: 10.1515/cclm-2017-0638. PMID: 29466232.

10. Selliah N, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, et al. 2019; Flow cytometry method validation protocols. Current Protocols in Cytometry. 87:e53. DOI: 10.1002/cpcy.53. PMID: 30418706.

11. Schmitt N, Bentebibel SE, Ueno H. 2014; Phenotype and functions of memory Tfh cells in human blood. Trends in Immunology. 35:436–42. DOI: 10.1016/j.it.2014.06.002. PMID: 24998903. PMCID: PMC4152409.

12. CLSI. Validation of assays performed by flow cytometry. Laboratory Standards Institute, 2021.
13. Hale JS, Ahmed R. 2015; Memory T follicular helper CD4 T cells. Frontiers in Immunology. 6:16. DOI: 10.3389/fimmu.2015.00016. PMID: 25699040. PMCID: PMC4313784.

Fig. 1
Gating strategy for Tfh cells and their subtypes. Tfh was used to define the populations of live CXCR5+ helper T cells. Three Tfh types are presented according to the positivity of CXCR3 and CCR6: types 1, 2, and 17.
Abbreviation: Tfh, follicular helper T.
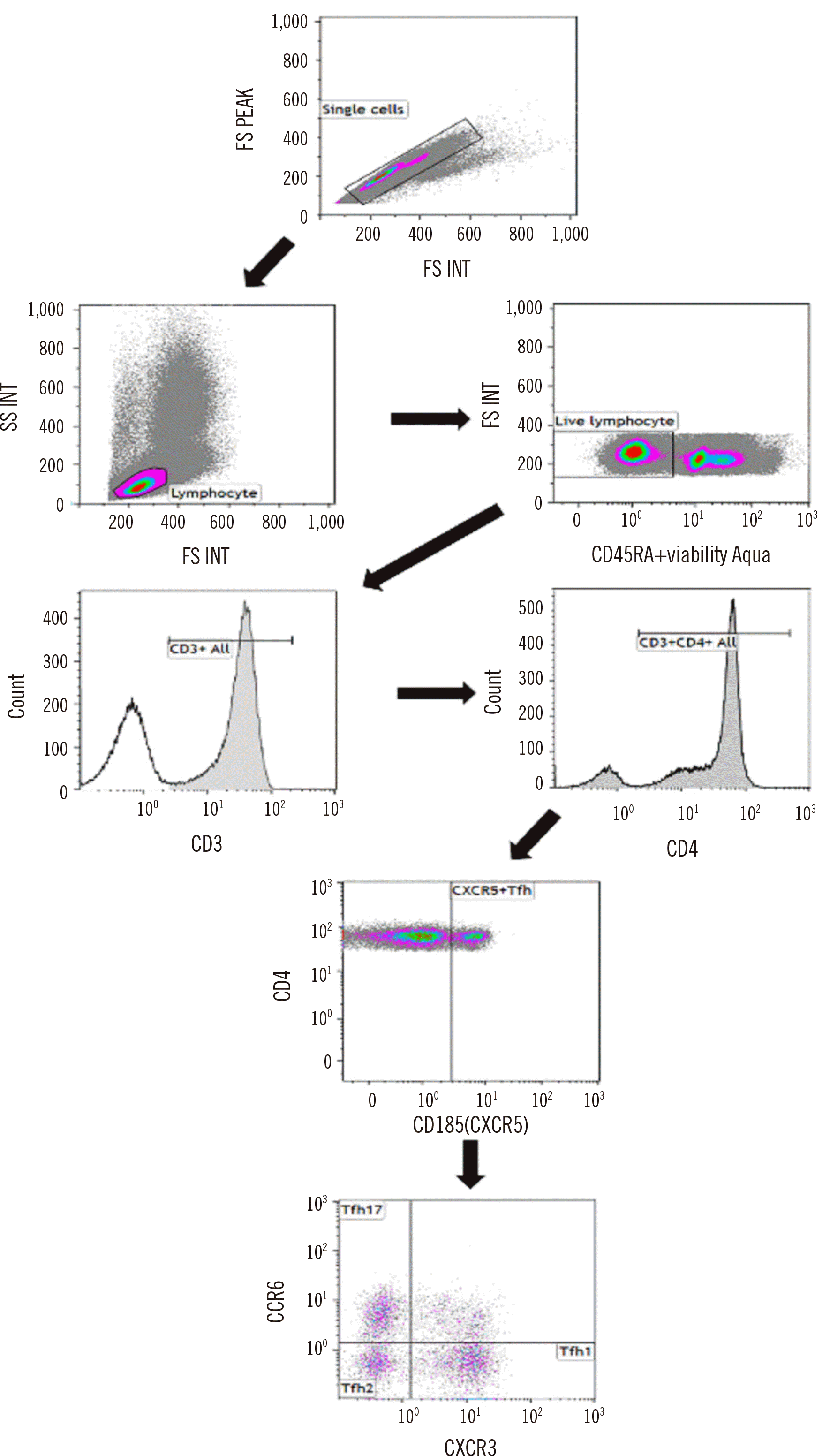
Fig. 2
Linearity of the follicular helper T cell measurement range for different serially diluted samples. R2, coefficient of determination.
Abbreviation: Tfh, follicular helper T-cells.
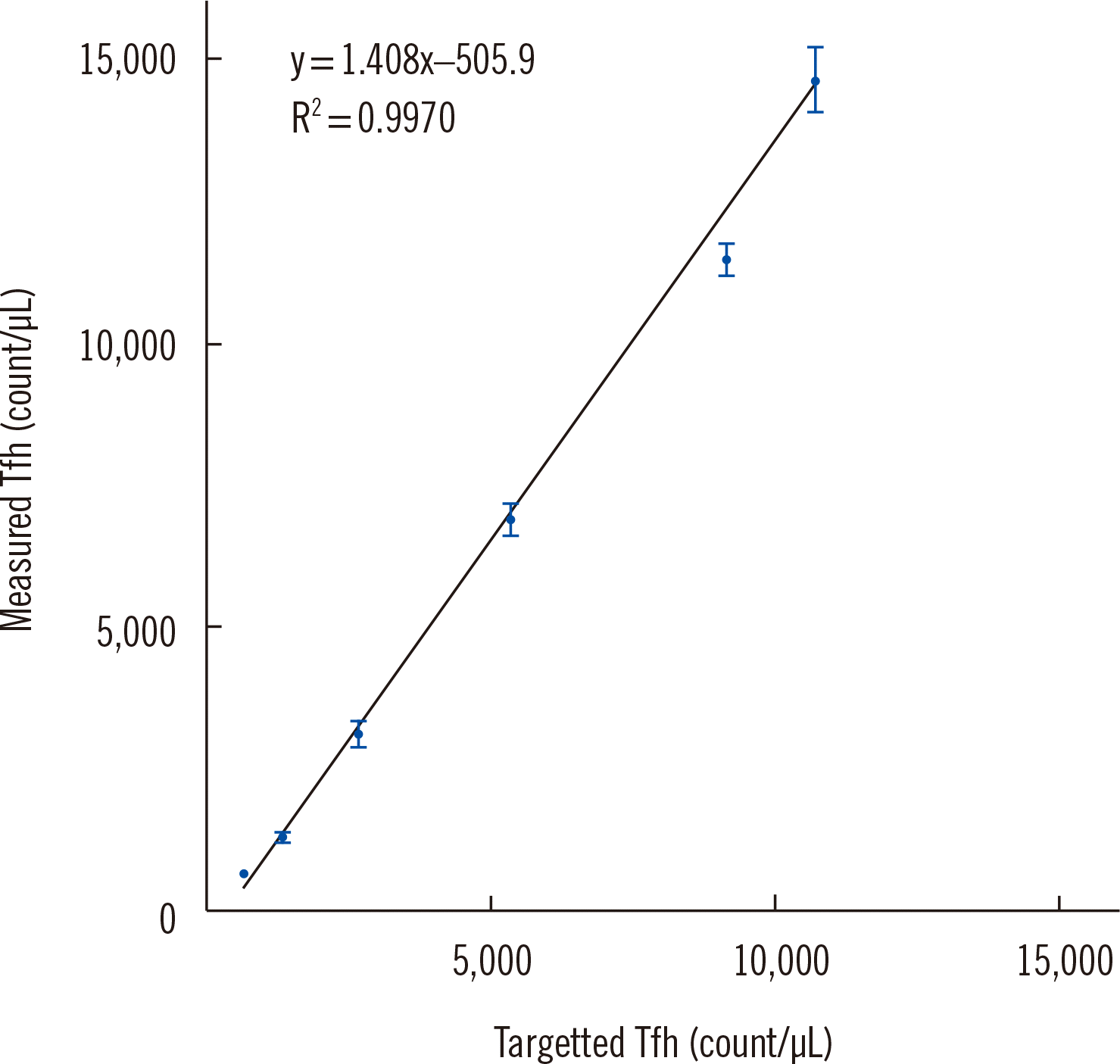
Table 1
Intra- and inter-assay precision for Tfh cells
| Precision | Counts of analysis | Absolute count of Tfh cells (/μL)* | %CV | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Tfh cells (%) | Tfh1 cells (%) | Tfh2 cells (%) | Tfh17 cells (%) | ||||
| Intra-assay | Sample-1 | 3 | 1,186 | 1.67 | 3.57 | 2.25 | 7.64 |
| Sample-2 | 3 | 130 | 0.56 | 0.82 | 1.82 | 1.34 | |
| Sample-3 | 3 | 29 | 1.29 | 0.21 | 4.68 | 0.90 | |
| Inter-assay | Sample-1 | 12 | 1,068 | 2.19 | 8.53 | 20.96 | 16.0 |
| Sample-2 | 12 | 128 | 3.13 | 4.99 | 10.17 | 4.50 | |
| Sample-3 | 12 | 29 | 6.51 | 7.13 | 6.94 | 9.48 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download