Abstract
Background
In non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR) mutation testing of tumor tissue should be conducted at diagnosis. Alternatively, circulating tumor DNA can be used to detect EGFR mutation. We compared the cost and clinical effect of three strategies according to the application of the EGFR test.
Methods
Decision models were developed to compare the cost-effectiveness of tissue-only, tissue-first, and plasma-first diagnostic strategies as first- and second-line treatments for NSCLC from the perspective of the Korean national healthcare payer. Progression-free survival (PFS), overall survival (OS), and direct medical costs were assessed. A one-way sensitivity analysis was performed.
Results
The plasma-first strategy correctly identified numerous patients in the first- and second-line treatments. This strategy also decreased the cost of biopsy procedures and complications. Compared with that when using the other two strategies, the plasma-first strategy increased PFS by 0.5 months. The plasma-first strategy increased OS by 0.9 and 1 month compared with that when using the tissue-only and tissue-first strategies, respectively. The plasma-first strategy was the least expensive first-line treatment but the most expensive second-line treatment. First-generation tyrosine kinase inhibitor and the detection rate of the T790M mutation in tissues were the most cost-influential factors.
Lung cancer is a common cause of death worldwide [1]. In Korea, lung cancer is the fourth most frequently diagnosed cancer (11% of all tumors) and the leading cause of cancer-related deaths [2]. In 2017, approximately 27,000 new cases of lung cancer and 17,980 lung cancer-related deaths were reported worldwide [2]. Despite advances in the early detection of lung cancer, most patients present with locally advanced or metastatic disease [3]. Consequently, these patients have a very poor prognosis. Tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, are used in the treatment of lung cancer. Sensitizing mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) gene predict the response to TKIs. The detection rate of EGFR mutations is approximately 30%–40% in NSCLC cases in Asia and approximately 2%–8% in cases in Western countries [4]. In up to two-thirds of cases, resistance to EGFR-TKIs is mediated by the T790M mutation, a secondary EGFR mutation acquired during EGFR-TKI treatment after a median of 8–16 months [5]. Osimertinib, a third-generation EGFR-TKI, is selective for the EGFR-sensitizing mutation and T790M resistance mutation [6]. Recent evidence-based guidelines recommend testing for EGFR mutations upon advanced NSCLC diagnosis to guide treatment decisions. The current standard clinical approach is the genotyping of tumor biopsy tissues. However, the feasibility of the biopsy (patient status and tumor location), risk of complications during biopsy, high cost (including procedural and adverse events), lengthy turnaround time, and tumor heterogeneity are limitations to the use of tissue biopsy for genetic analysis [7]. Circulating tumor DNA (ctDNA) released from tumor cells is present in the blood of patients with advanced NSCLC [8, 9]. Plasma ctDNA or urine testing offers a minimally invasive or noninvasive alternative for detecting the EGFR mutation status [9]. EGFR mutation profiles obtained from liquid biopsy are now accepted for use in treatment decisions for patients with NSCLC. In addition to tumor EGFR genotyping, plasma EGFR genotyping is covered by the Korea National Health Insurance Service (KNHIS) since May 2018. However, insurance coverage is applied only in very limited cases, such as when it is not possible to collect tissue, and is limited to two uses per patient.
With the development of medical technology and new drugs, treatment methods for cancer are diversifying and standard treatment guidelines are being revised rapidly. Consequently, the financial burden on healthcare payers and patients has increased. Therefore, efficient resource allocation must be considered. Although the plasma EGFR test is covered by the KNHIS, a systematic cost-effectiveness analysis has never been conducted. Economic evaluations of individual diagnostic and treatment strategies have been sporadic. As in previous studies, the mutation detection rate and cost data depend on the target population; accordingly, the results can vary by country. We aimed to evaluate the economic and clinical impact of three diagnostic strategies on the initial diagnosis and disease progression of advanced and metastatic NSCLC based on the KNHIS from the payer’s perspective.
Decision models were developed to compare the cost-effectiveness of the three diagnostic strategies for the first- and second-line treatment of NSCLC from the perspective of Korean national healthcare providers (Fig. 1). The tissue-only strategy provides a test for the EGFR mutation status using only tissue samples. No additional diagnostic investigations are performed for biopsies with insufficient tissue or “indeterminate” results. The tissue-first strategy uses tissue biopsies for diagnostic investigations. Unlike in the tissue-only strategy, a liquid biopsy is performed in cases with undetermined tissue biopsy results. The KNHIS covers this strategy. The plasma-first strategy involves the use of a liquid biopsy for all eligible patients. If the outcome of the liquid biopsy is negative, an EGFR mutation test is performed using the remaining tissue collected for diagnosis or tissue from a re-biopsy. These three strategies were identically applied for initial diagnosis and during disease progression, except in cases of acquired resistance, where a repeat biopsy was performed owing to greater tumor heterogeneity compared to that in newly diagnosed patients [10]. In our model, patients with sensitizing mutations received a first-generation TKI (gefitinib) until disease progression. Patients without sensitizing mutations were considered for platinum therapy (carboplatin and paclitaxel), identical to the regimen used in the Iressa Pan-Asia Study (IPASS) trial (N=1,217) [11, 12]. Patients with T790M-positive disease received a third-generation TKI (osimertinib) until disease progression. Patients with T790M-negative disease were considered for platinum therapy (carboplatin and pemetrexed), identical to the regimen used in the A Phase III, Open Label, Randomized Study of AZD9291 Versus Platinum-Based Doublet Chemotherapy for Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer Whose Disease Has Progressed With Previous Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Whose Tumours Harbour a T790M Mutation Within the Epidermal Growth Factor Receptor Gene (AURA3) trial (N=419) [13-15].
The patient population for this analysis was based on the characteristics of the patients enrolled in the IPASS trial for first-line treatment and in the AURA3 trial for second-line treatment. The IPASS trial consisted of patients ≥18 years of age who had histologically confirmed stage IIIB or IV NSCLC with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and who had no history of chemotherapy. The AURA3 trial consisted of patients ≥18 years of age with an ECOG performance status score (which reflects functional status) of 0 or 1 who experienced progression on EGFR-TKI therapy for EGFR-positive NSCLC with the T790M mutation and who were eligible for subsequent therapy.
Test results were undetermined in 10% of patients. These cases were selected based on expert opinions and were considered comparable to the results of another study [16]. The frequencies of sensitizing and T790M mutations were calculated as the averages of the frequencies in previous studies that analyzed the EGFR mutation status using PCR methods with tissue and plasma in advanced (stage IIIB, IV) NSCLC [5, 10, 15, 17- 19]. The values and ranges of the input parameters are listed in Table 1. The number of patients receiving first-line treatment for sensitizing mutations was calculated as the conditional probability based on the decision tree in Fig. 1 and the values in Table 1.
We constructed a Markov model with three health states to analyze progression-free survival (PFS), progressive disease, and death. Patients could move from one state to another during each three-week cycle. Event (disease progression and post-progression mortality) probabilities were based on published clinical trial data. A time horizon of 4 years was adopted to reflect the limited remaining life of the patients. The median overall survival (OS) and PFS after second-line treatment were calculated by estimating the rates of daily progression and mortality using the Kaplan–Meier method [11, 13].
We considered only reimbursed direct medical costs, including the costs of biopsy, EGFR testing, drugs and administration, and hospital administration due to complications, based on the health insurance claims data of secondary hospitals (Table 1). We assumed that patients who underwent bronchoscopic biopsy or computed tomography-guided percutaneous biopsy were hospitalized for three days. Tissue and plasma EGFR genotyping are covered by the national insurance system. The costs of both types of EGFR genotyping are 131,505 KRW. Pneumothorax is the most common complication of needle biopsy of the lungs and occurs in approximately 20% of patients [20-22]. Pneumothorax requiring a chest tube is needed for 4%–7% of patients [20-22], which was estimated to be 6% in this study. The cost of hospital admission for pneumothorax after tissue biopsy was assumed to be 917,775 KRW for a stay of five days. We considered drug costs based on drug reimbursement, price lists, and the administration of intravenous therapies. To calculate the cost of each drug, we assumed a body surface area of 1.73 m2 and a glomerular filtration rate of 93 mL/min based on the median age of patients in the IPASS and AURA3 trials, respectively. The total cost of the first-line treatment was calculated as the sum of all products of the conditional probabilities shown in Fig. 1 and the cost of each item. The total cost of second-line treatment was calculated considering the progression and death statuses using the Markov chain model as the total cost over 4 years.
Several sensitivity analyses were conducted to evaluate the uncertainties and robustness of the models. A one-way sensitivity analysis was performed to explore how uncertainty in the input parameters influenced the outcomes. The key parameters were detection rates of EGFR-sensitizing and T790M mutations in tissue and plasma, complication rate of biopsies, cost of biopsy procedures, hospitalization due to complications, and first-generation TKI. The input values of the epidemiological data varied between the ranges identified in published studies. Costs related to hospitalization varied depending on whether the hospital was a tertiary hospital or comprised a type of shared room.
The results of the base-case analysis with a 4-year time horizon, as well as the economic and health outcomes estimated using the model, are shown in Table 2. Considering both the first and second lines of treatment, the plasma-first strategy exhibited the highest mutation detection rate. The plasma-first strategy was expected to detect 96.8% and 91.7% of patients with sensitizing and T790M mutations, respectively. The identification of sensitizing mutations and TKI treatment improved with the plasma-first strategy (+12.2% vs. tissue-only strategy; +4.3% vs. tissue-first strategy). The identification of T790M mutations and third-generation TKI treatment improved with the plasma-first strategy (+8.5% vs. tissue-only strategy; +9.5% vs. tissue-first strategy). The plasma-first strategy reduced the costs of biopsy and associated complications. However, the plasma-first strategy slightly increased the cost of molecular testing. For first-line treatment, the plasma-first strategy is expected to have the lowest total cost.
PFS was 9.6 months for the plasma-first strategy and 9.1 months for the tissue-only and tissue-first strategies. OS was also expected to increase slightly using the plasma-first strategy. For second-line treatment, the plasma-first strategy increases the cost.
The results of the one-way sensitivity analyses are presented in a tornado diagram (Fig. 2). At the time of first diagnosis, the plasma-first strategy exhibited cost-saving effects within a range of input values. For all three strategies, the first-generation TKI drug cost had the greatest influence on the total cost, with gefitinib being the most cost-effective. The second most influential variable in reducing the expected cost was the biopsy cost (according to room selection) in the tissue-only and tissue-first strategies and the detection rate of sensitizing mutations in plasma in the plasma-first strategy.
During progression, the tissue-first strategy provided cost savings over a range of input values. For all three strategies, the detection rate of the T790M mutation in tissues had the greatest influence on the total cost. The second most influential variable in reducing the expected cost was the T790M mutation detection rate in plasma for the tissue-first and plasma-first strategies and the biopsy cost in the tissue-only strategy.
This study is the first to examine the clinical and economic impact of three diagnostic strategies for the first- and second-line treatment of advanced and metastatic NSCLC from the perspective of the Korean healthcare payer. Traditionally, molecular analysis is performed on tumor tissues. However, tissue biopsies are invasive and have a high risk of complications [7]. Biopsy during disease progression is even more problematic (e.g., an unfavorable patient condition and shrunken tumors). Although the KNHIS covers ctDNA testing for EGFR mutations in advanced NSCLC since 2018, ctDNA testing can only be performed once when changing drugs in situations where a biopsy is not feasible. Recently, the National Comprehensive Cancer Network recommended the use of a plasma EGFR test as a screening method to detect T790M, irrespective of the feasibility of repeated tissue biopsies [23]. Additionally, the plasma-first approach is preferred for the evaluation of acquired resistance, according to the consensus statement of the International Association for the Study of Lung Cancer [24].
Our analysis showed that the plasma-first strategy (compared with the tissue-only and tissue-first strategies) is expected to increase the detection of sensitizing or T790M mutations and related treatment with TKIs. This was because of the greater use of ctDNA testing, which resulted in a significant increase in the number of identified mutation cases when using the plasma-first strategy. These results are comparable with those of a previous study [25]. Due to heterogeneity, the T790M status of individual samples may not represent the overall T790M status of the disease [9]. In the AURA extension and AURA2 phase II studies, 27 (4.9%) patients were identified as T790 mutation-negative through tissue testing and T790M mutation-positive through plasma testing [17]. Our study confirmed that the target of treatment for NSCLC could be diagnosed more accurately using the plasma EGFR test. Our results support the need to expand the application of ctDNA testing in Korea. This improvement in outcomes is a consequence of the few patients with T790M-positive tumors that received indeterminate or false-negative test results based on the tissue test but were likely to benefit from targeted third-generation TKIs.
The median survival is more than 2 years longer in patients with advanced stage IV NSCLC with EGFR-sensitizing mutations than in those with wild-type EGFR [26, 27]. It is important to accurately identify patients who should receive targeted therapy because a significant proportion of the side effects of targeted therapy can be controlled, compared with those of cytotoxic anticancer drugs; thus, targeted therapy can be administered even to elderly patients or patients with relatively poor systemic conditions, and favorable outcomes are expected.
The number of biopsies was reduced in both first- and second-line treatments using the plasma-first strategy, and the cost associated with complications was also reduced. In first-line treatment, the plasma-first strategy had the lowest total cost of care; however, in second-line treatment, the plasma-first strategy had the highest total cost of care. The cost per patient increased with the plasma-first strategy because of the higher use of third-generation TKIs in patients with T790M-positive disease and the increased survival rate.
This study had several limitations. First, costs vary by circumstance, which complicates the collection of data regarding specific costs and complications. The effect was evaluated through a sensitivity analysis, and the results should be interpreted with caution. Second, the trial-based model does not fully simulate the natural course of the disease in the real world. The regimens used for disease treatment vary among physicians. The results may not adequately reflect efficacy and resource utilization in routine clinical practice. Third, an important assumption underlying this model is that the testing led to different treatments that did not consider other factors that could contribute to variations in the outcome of therapy, such as the availability of test results. Delays in tissue biopsies often occur because of scheduling and laboratory processing times. The median turnaround time (TAT) from ordering a biopsy to receiving the results was 12 (1–54) days for patients with newly diagnosed NSCLC and 27 (1–146) days for patients with acquired resistance [28]. The median TAT for blood-based mutation testing was 3 (1–7) days [28]. An evaluation of other EGFR mutation testing approaches and potential treatments is needed. In our sensitivity analyses, the T790M detection rates in tissue and plasma had significant influences on the results of second-line treatment. Only the real-time PCR detection rate was considered. However, many highly sensitive and specific platforms are available, including real-time quantitative PCR, peptide nucleic acid-locked nucleic acid clamp, beads, emulsion, amplification, and magnetics, digital PCR (dPCR), and next-generation sequencing (NGS) [7, 29]. According to a previous study [30] that used Cobas tissue test results as a reference, the percent agreement with plasma T790M positivity was 51% (110 out of 215 samples) for Cobas plasma, 58% (110 out of 189 samples) for dPCR, and 66% (136 out of 207 samples) for NGS. In addition, more accurate disease diagnosis is possible when using a sensitive genotyping method combined with mutation enrichment technologies [31, 32]. Because ctDNA exists in a small proportion in the blood and is very unstable, both the test method and pre-analytical variables have important influences on the results [33]. While this study was in progress, osimertinib, a third-generation TKI, was approved as a first-line treatment for patients with advanced EGFR-mutated NSCLC. The United States, Germany, Italy, Switzerland, the United Kingdom, France, Japan, and other countries worldwide are now using osimertinib as a standard treatment. However, this treatment has not been approved by the Korean insurance system. Therefore, further study on new treatment options is required.
In conclusion, the plasma-first strategy can decrease costs and morbidities compared to tissue-based EGFR mutation testing. We confirmed that candidates for targeted treatment of NSCLC can be more accurately identified by plasma EGFR testing. The selection of an appropriate diagnostic strategy is important for optimal primary and secondary treatment of advanced NSCLC. It is necessary to use an appropriate test method for each genetic analysis so that the optimal treatment for each patient can be administered in the shortest time.
Notes
AUTHOR CONTRIBUTIONS
Cho SM, Kim Y, and Lee KA conceptualized and designed the study. Cho SM collected data and wrote the manuscript. Lee HS and Jeon SY performed statistical analyses. Lee JK and Kong SY were involved in the data collection. Kim YJ discussed the data. Lee KA supervised the study and reviewed the manuscript. All authors have read and approved the final manuscript.
REFERENCES
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. 2015; Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–86. DOI: 10.1002/ijc.29210. PMID: 25220842.

2. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2017. Ministry of Health and Welfare;2020.
3. Lim C, Sung M, Shepherd FA, Nouriany N, Sawczak M, Paul T, et al. 2016; Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J Thorac Oncol. 11:79–84. DOI: 10.1016/j.jtho.2015.09.006. PMID: 26762742.

4. Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. 2005; Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 23:5900–9. DOI: 10.1200/JCO.2005.02.857. PMID: 16043828.

5. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. 2013; Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 19:2240–7. DOI: 10.1158/1078-0432.CCR-12-2246. PMID: 23470965. PMCID: PMC3630270.
6. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. 2014; AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 4:1046–61. DOI: 10.1158/2159-8290.CD-14-0337. PMID: 24893891. PMCID: PMC4315625.

7. Goldman JW, Noor ZS, Remon J, Besse B, Rosenfeld N. 2018; Are liquid biopsies a surrogate for tissue EGFR testing? Ann Oncol. 29(Suppl 1):i38–i46. DOI: 10.1093/annonc/mdx706. PMID: 29462257.
8. Goto K, Ichinose Y, Ohe Y, Yamamoto N, Negoro S, Nishio K, et al. 2012; Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 7:115–21. DOI: 10.1097/JTO.0b013e3182307f98. PMID: 21900837.

9. Diaz LA Jr. 2014; and Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 32:579–86. DOI: 10.1200/JCO.2012.45.2011. PMID: 24449238. PMCID: PMC4820760.
10. Karlovich C, Goldman JW, Sun JM, Mann E, Sequist LV, Konopa K, et al. 2016; Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res. 22:2386–95. DOI: 10.1158/1078-0432.CCR-15-1260. PMID: 26747242. PMCID: PMC6886231.
11. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. 2009; Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361:947–57. DOI: 10.1056/NEJMoa0810699. PMID: 19692680.

12. Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. 2011; Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 29:2866–74. DOI: 10.1200/JCO.2010.33.4235. PMID: 21670455.

13. Wu Y-L, Mok TSK, Han J-Y, Ahn M-J, Delmonte A, Ramalingam SS, et al. 2019; Overall survival (OS) from the AURA3 phase III study: osimertinib vs platinum-pemetrexed (plt-pem) in patients (pts) with EGFR T790M advanced non-small cell lung cancer (NSCLC) and progression on a prior EGFR-tyrosine kinase inhibitor (TKI). Ann Oncol. 30:IX158. DOI: 10.1093/annonc/mdz437.001.
14. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. 2017; Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 376:629–40. DOI: 10.1056/NEJMoa1612674. PMID: 27959700. PMCID: PMC6762027.

15. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. 2016; Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 34:3375–82. DOI: 10.1200/JCO.2016.66.7162. PMID: 27354477. PMCID: PMC5035123.

16. Sands J, Li Q, Hornberger J. 2017; Urine circulating-tumor DNA (ctDNA) detection of acquired EGFR T790M mutation in non-small-cell lung cancer: an outcomes and total cost-of-care analysis. Lung Cancer. 110:19–25. DOI: 10.1016/j.lungcan.2017.05.014. PMID: 28676213.

17. Jenkins S, Yang JCH, Ramalingam SS, Yu K, Patel S, Weston S, et al. 2017; Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 12:1061–70. DOI: 10.1016/j.jtho.2017.04.003. PMID: 28428148.
18. Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, et al. 2015; EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 90:509–15. DOI: 10.1016/j.lungcan.2015.10.004. PMID: 26494259.

19. Goldman JW, Karlovich C, Sequist LV, Melnikova V, Franovic A, Gadgeel SM, et al. 2018; EGFR genotyping of matched urine, plasma, and tumor tissue in patients with non-small-cell lung cancer treated with rociletinib, an EGFR tyrosine kinase inhibitor. JCO Precis Oncol. 2:1–13. DOI: 10.1200/PO.17.00116. PMID: 35135111.
20. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. 2017; Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 27:138–48. DOI: 10.1007/s00330-016-4357-8. PMID: 27108299. PMCID: PMC5127875.

21. Covey AM, Gandhi R, Brody LA, Getrajdman G, Thaler HT, Brown KT. 2004; Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 15:479–83. DOI: 10.1097/01.RVI.0000124951.24134.50. PMID: 15126658.

22. Wiener RS, Schwartz LM, Woloshin S, Welch HG. 2011; Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 155:137–44. DOI: 10.7326/0003-4819-155-3-201108020-00003. PMID: 21810706. PMCID: PMC3150964.

23. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN clinical practice guidelines in oncology. Non-small cell lung cancer, Version 5. 2021.
24. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. 2021; Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 16:1647–62. DOI: 10.1016/j.jtho.2021.06.017. PMID: 34246791.

25. Gancitano G, Ravasio R, Dionisi M, Cortinovis D. Cost-consequence analysis of three different diagnostic strategies in the first- and second-line treatment of locally advanced or metastatic non-small-cell lung cancer. Farmeconomia, Health Economics and Therapeutic Pathways. 2018; 19:DOI: 10.7175/fe.v19i1.1354.

26. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. 2010; Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 362:2380–8. DOI: 10.1056/NEJMoa0909530. PMID: 20573926.

27. Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. 2013; Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 24:54–9. DOI: 10.1093/annonc/mds214. PMID: 22967997.
28. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, et al. 2016; Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2:1014–22. DOI: 10.1001/jamaoncol.2016.0173. PMID: 27055085. PMCID: PMC4982795.

29. Sorber L, Zwaenepoel K, Deschoolmeester V, Van Schil PE, Van Meerbeeck J, Lardon F, et al. 2017; Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer. 107:100–7. DOI: 10.1016/j.lungcan.2016.04.026. PMID: 27180141.

30. Papadimitrakopoulou VA, Han JY, Ahn MJ, Ramalingam SS, Delmonte A, Hsia TC, et al. 2020; Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer. 126:373–80. DOI: 10.1002/cncr.32503. PMID: 31769875.

31. Kim B, Kim Y, Shin S, Lee ST, Cho JY, Lee KA. 2022; Application of CRISPR/Cas9-based mutant enrichment technique to improve the clinical sensitivity of plasma EGFR testing in patients with non-small cell lung cancer. Cancer Cell Int. 22:82. DOI: 10.1186/s12935-022-02504-2. PMID: 35168603. PMCID: PMC8845274.
32. Kim Y, Shin S, Lee KA. 2021; Exosome-based detection of EGFR T790M in plasma and pleural fluid of prospectively enrolled non-small cell lung cancer patients after first-line tyrosine kinase inhibitor therapy. Cancer Cell Int. 21:50. DOI: 10.1186/s12935-021-01761-x. PMID: 33435996. PMCID: PMC7802208.
33. Shin S, Woo HI, Kim JW, Kim Y, Lee KA. 2022; Clinical practice guidelines for pre-analytical procedures of plasma epidermal growth factor receptor variant testing. Ann Lab Med. 42:141–9. DOI: 10.3343/alm.2022.42.2.141. PMID: 34635607. PMCID: PMC8548242.

Fig. 1
Schematic representation of the diagnostic strategies analyzed in this study. (A) First-line treatment (B) Second-line treatment.
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; 3rd-gen, third-generation.
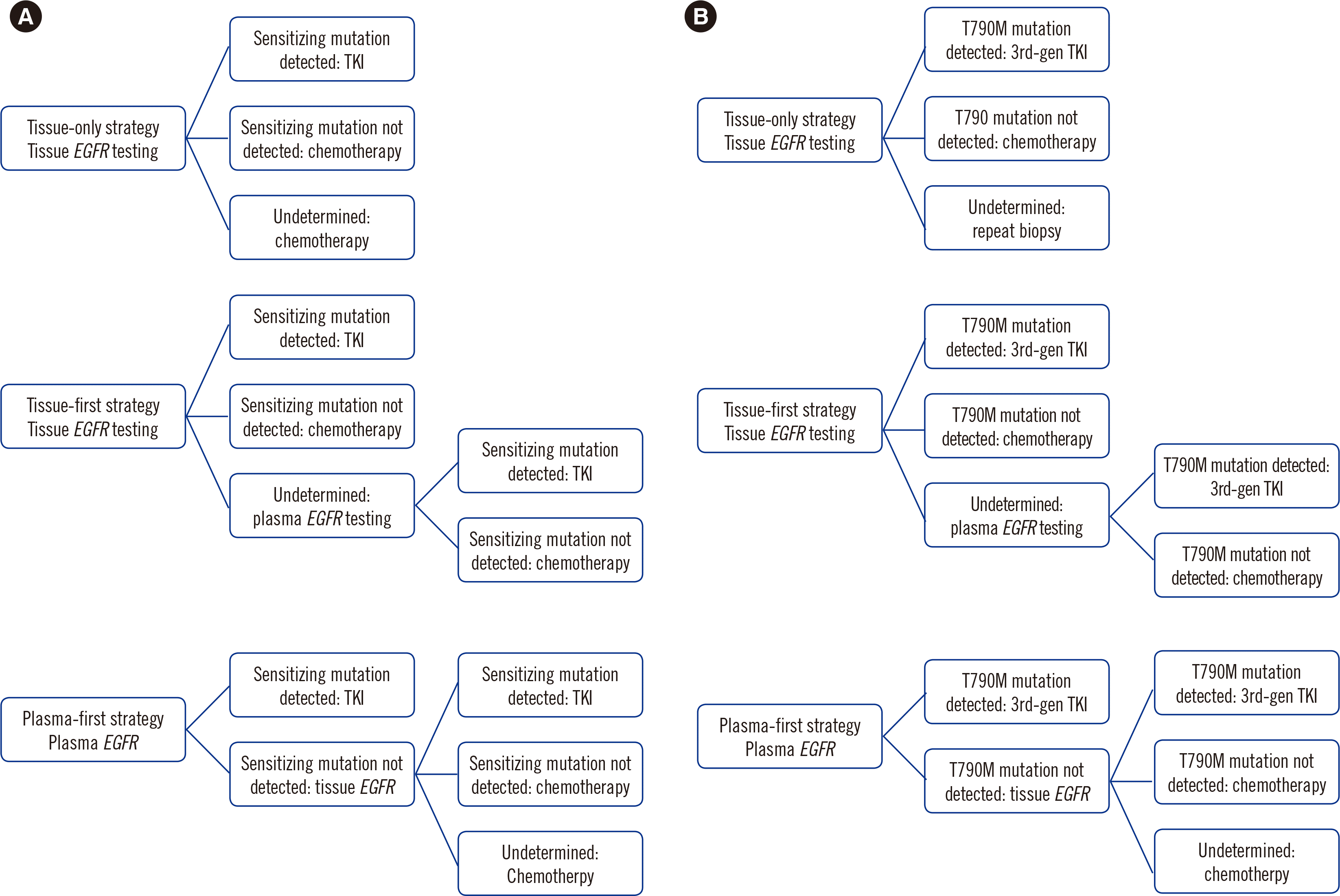
Fig. 2
Tornado diagram of sensitivity analysis. (A) Tissue-only strategy. (B) Tissue-first strategy. (C) Plasma-first strategy in first-line treatment. (D) Tissue-only strategy. (E) Tissue-first strategy. (F) Plasma-first strategy in second-line treatment. The horizontal bars in the tornado diagrams indicate how wide the variation in the total cost due to a change in a given input is. At the time of first diagnosis, first-generation TKI is the most cost-influential factor. At the time of disease progression, T790M status in tumor tissue is the most cost-influential factor.
Abbreviations: 1st-gen, first-generation; TKI, tyrosine kinase inhibitor.
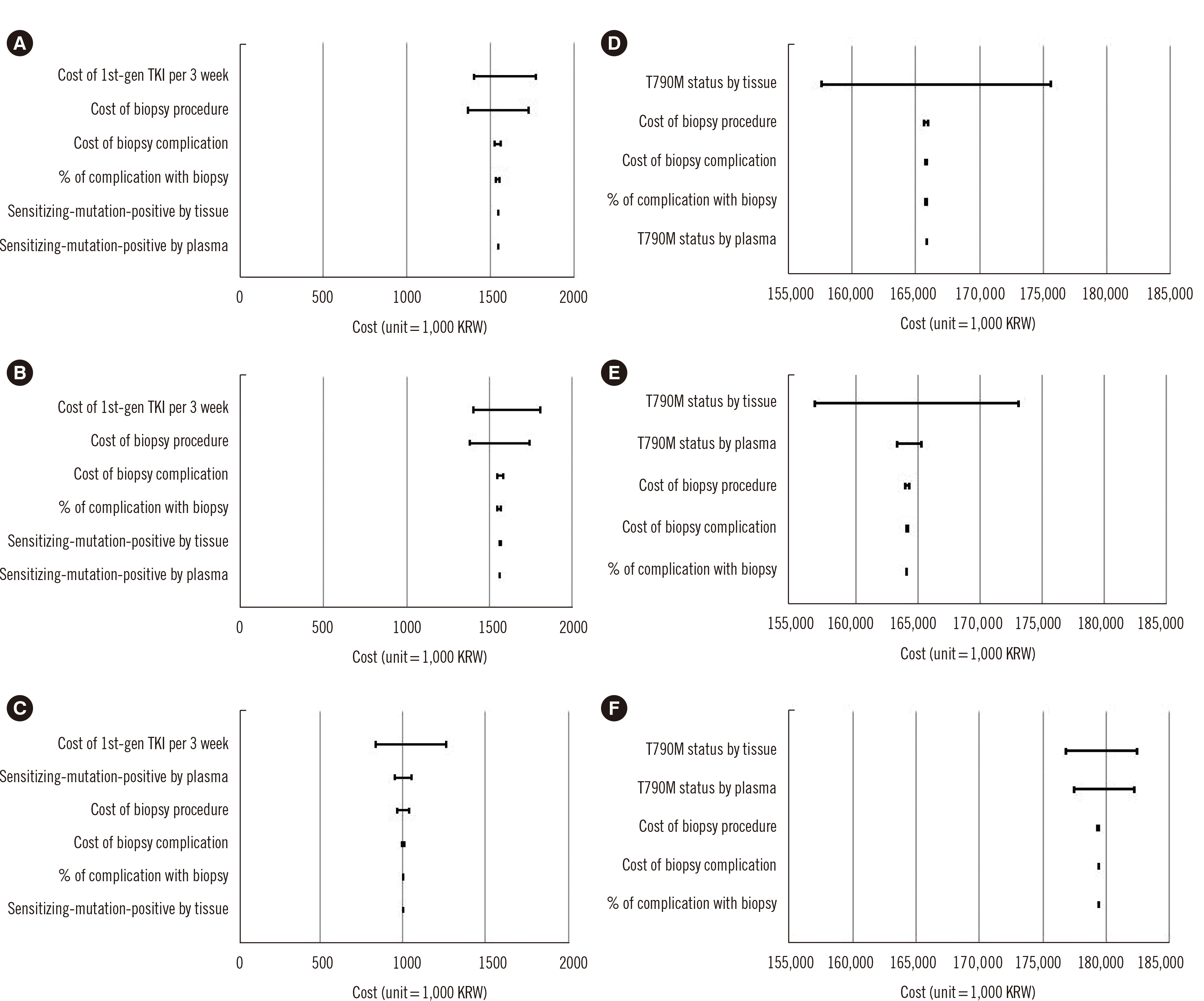
Table 1
Values and ranges of input parameters in the analysis
| Input parameter | Value | Range | Source |
|---|---|---|---|
| Sensitizing mutation status in tissue (%) | |||
| Positive | 84.6 | 83.7–87.5 | [10, 17, 18] |
| Negative | 5.4 | 1.5–6.3 | [10, 17, 18] |
| Indeterminate | 10.0 | 10.0–11.0 | [16] |
| Sensitizing mutation status in plasma (%) | |||
| Positive | 79.2 | 73.3–85.0 | [10, 17, 18] |
| Negative | 20.9 | 8.2–22.4 | [10, 17, 18] |
| Complication of biopsy | 6.0 | 4.3–6.8 | [20–22] |
| T790M status by tissue (%) | |||
| Positive | 75.6 | 71.1–81.0 | [5, 15, 19] |
| Negative | 14.4 | 9.0–18.9 | [5, 15, 19] |
| Indeterminate | 10.0 | 10.0 | [5, 15, 19] |
| T790M status by plasma (%) | |||
| Positive | 65.9 | 61.0–73.2 | [5, 15, 19] |
| Negative | 34.1 | 26.8–39.0 | [5, 15, 19] |
| Cost (KRW) | |||
| Biopsy procedure | 676,066 | 494,836–857,296 | |
| Hospitalization due to complication | 917,775 | 615,725–1,219,825 | |
| EGFR testing | 131,505 | ||
| Drug cost | |||
| First-generation TKI per day | 33,149 | 24,950–45,810 | |
| First-line chemotherapy per cycle | 596,132 | ||
| Third-generation TKI per day | 227,356 | ||
| Second-line chemotherapy per cycle | 926,609 | ||
| Subsequent treatment per cycle | 830,264 |
Table 2
Summary of the cost and outcome results in the analysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download