1. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019; 39 Suppl 1:19–31.

2. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018; 7:52.

3. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. Cham: Springer International Publishing;2018.
4. Chen Y, Weng S. Reappraisal of the T category for solitary intrahepatic cholangiocarcinoma by tumor size in 611 early-stage (T1-2N0M0) patients after hepatectomy: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Gastrointest Surg. 2021; 25:1989–99.

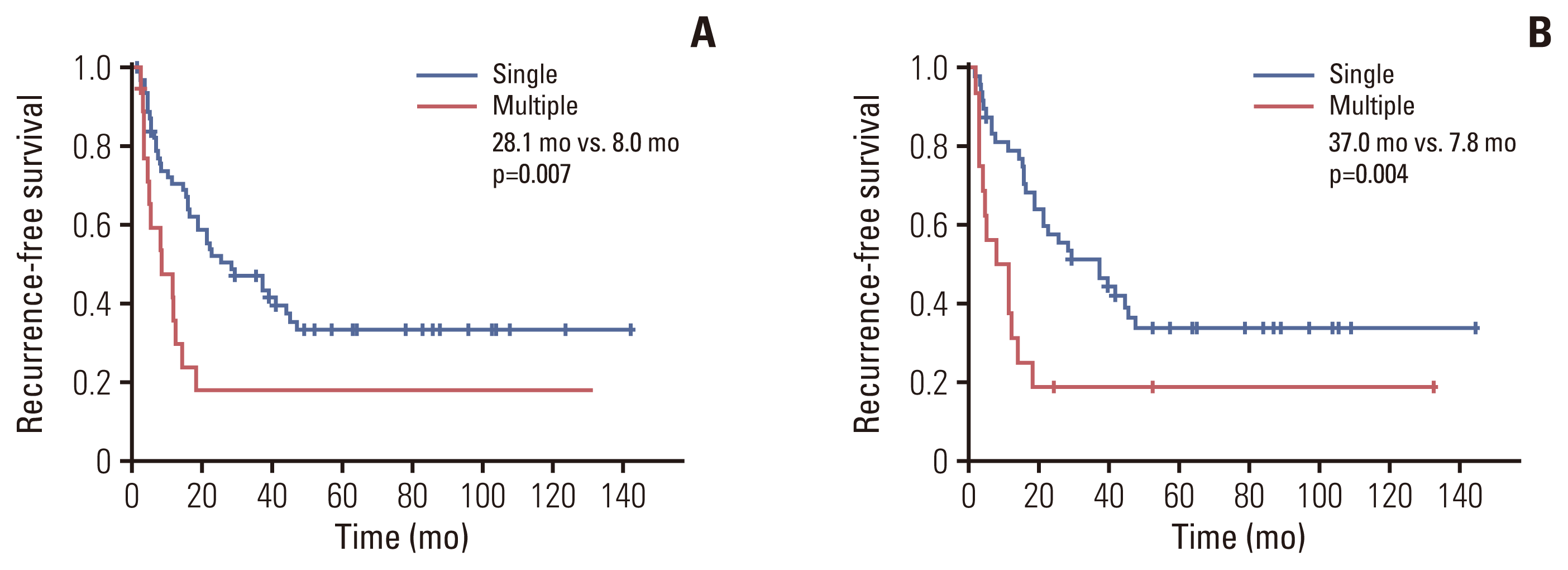
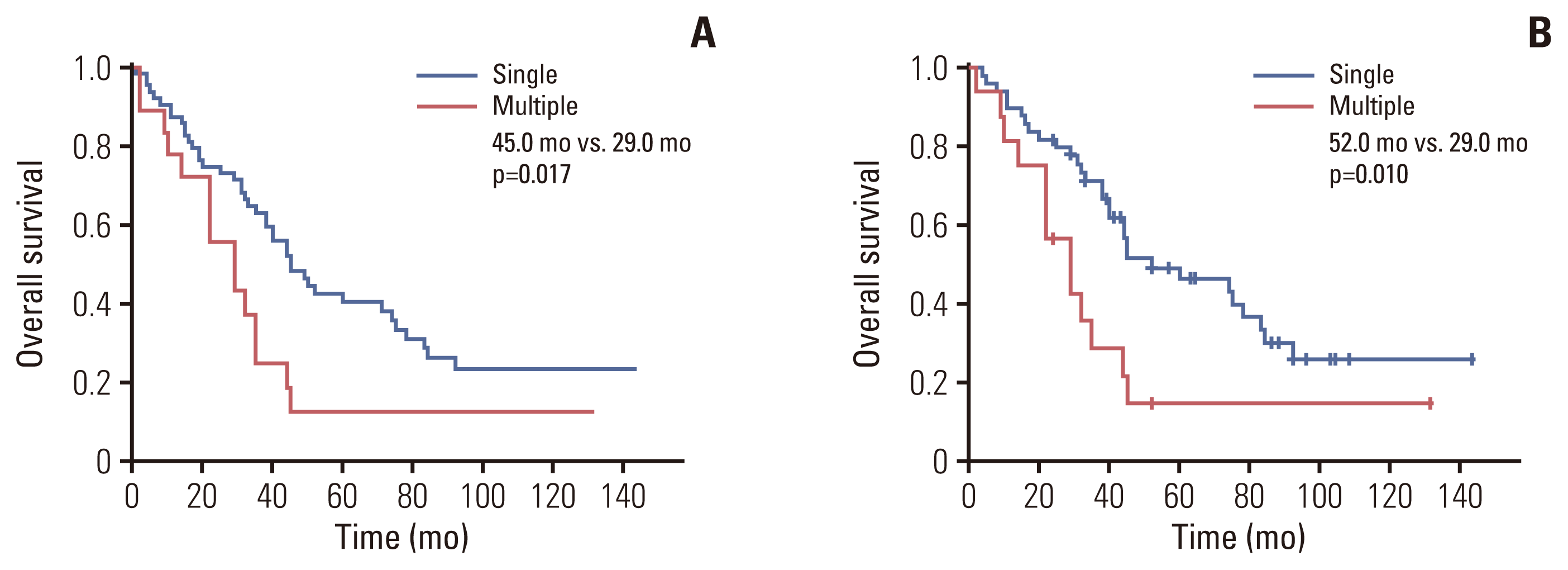
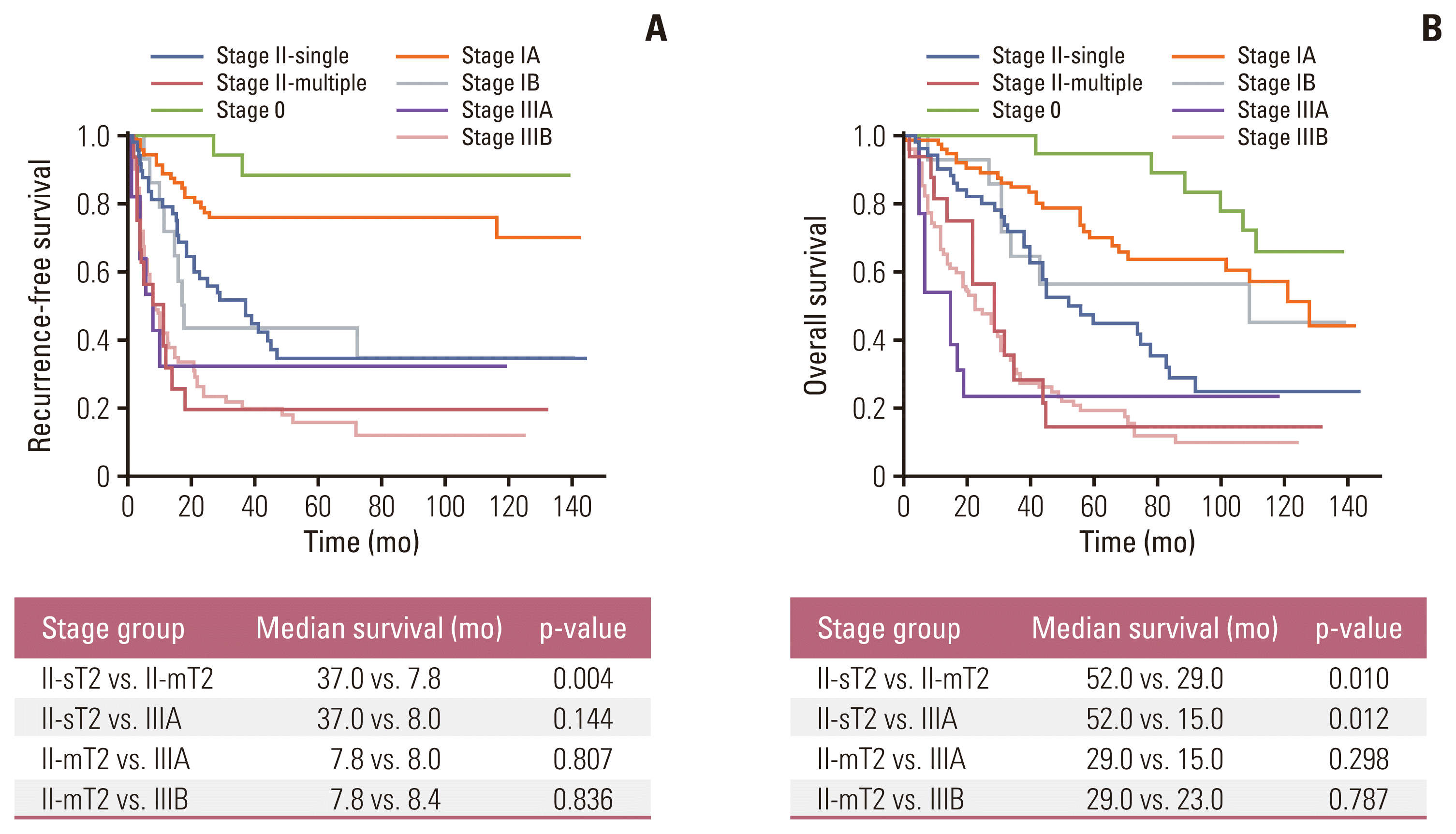
5. Turner KM, Delman AM, Kharofa J, Olowokure O, Sohal D, Quillin RC, et al. A national assessment of T2 staging for intrahepatic cholangiocarcinoma and the poor prognosis associated with multifocality. Ann Surg Oncol. 2022; 29:5094–102.

6. Conci S, Ruzzenente A, Vigano L, Ercolani G, Fontana A, Bagante F, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018; 25:3719–27.

7. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2009.
8. Lamarca A, Santos-Laso A, Utpatel K, La Casta A, Stock S, Forner A, et al. Liver metastases of intrahepatic cholangiocarcinoma: implications for an updated staging system. Hepatology. 2021; 73:2311–25.

9. Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, et al. The evaluation of the eighth edition of the AJCC/UICC staging system for intrahepatic cholangiocarcinoma: a proposal of a modified new staging system. J Gastrointest Surg. 2020; 24:786–95.

10. Wright GP, Perkins S, Jones H, Zureikat AH, Marsh JW, Holtzman MP, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. 2018; 25:83–90.

11. Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015; 17:669–80.

12. Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann Surg Oncol. 2020; 27:1372–84.

13. Uenishi T, Kubo S, Yamazaki O, Yamada T, Sasaki Y, Nagano H, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008; 15:417–22.
14. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020; 112:200–10.

15. Conci S, Campagnaro T, Danese E, Lombardo E, Isa G, Vitali A, et al. Role of inflammatory and immune-nutritional prognostic markers in patients undergoing surgical resection for biliary tract cancers. Cancers (Basel). 2021; 13:3594.

16. Yoon SJ, Park B, Kwon J, Lim CS, Shin YC, Jung W, et al. Development of nomograms for predicting prognosis of pancreatic cancer after pancreatectomy: a multicenter study. Biomedicines. 2022; 10:1341.

17. Miyahara Y, Takashi S, Shimizu Y, Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J Surg Oncol. 2020; 18:78.

18. Steele CW, Jamieson NB, Evans TR, McKay CJ, Sansom OJ, Morton JP, et al. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br J Cancer. 2013; 108:997–1003.

19. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003; 6:283–7.

20. Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: effects of advanced liver disease. Cancer Med. 2019; 8:5916–29.
21. Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB (Oxford). 2020; 22:1667–74.

22. Noguchi D, Kuriyama N, Nakagawa Y, Maeda K, Shinkai T, Gyoten K, et al. The prognostic impact of lymphocyte-to-C-reactive protein score in patients undergoing surgical resection for intrahepatic cholangiocarcinoma: a comparative study of major representative inflammatory/immunonutritional markers. PLoS One. 2021; 16:e0245946.
23. Tsilimigras DI, Mehta R, Aldrighetti L, Poultsides GA, Maithel SK, Martel G, et al. Development and validation of a Laboratory Risk Score (LabScore) to predict outcomes after resection for intrahepatic cholangiocarcinoma. J Am Coll Surg. 2020; 230:381–91.

24. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016; 18:79–87.

25. Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018; 105:848–56.
26. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019; 39(Suppl 1):143–55.
27. Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: a multicenter retrospective study. BMC Surg. 2021; 21:359.

28. Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Extent of lymph node dissection for accurate staging in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2022; 26:70–6.

29. Ruzzenente A, Conci S, Vigano L, Ercolani G, Manfreda S, Bagante F, et al. Role of lymph node dissection in small (≤ 3 cm) intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019; 23:1122–9.
30. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005; 29:728–33.

31. Sahara K, Tsilimigras DI, Merath K, Bagante F, Guglielmi A, Aldrighetti L, et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: which patients benefit the most from nodal evaluation? Ann Surg Oncol. 2019; 26:2959–68.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download