1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.

2. Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006; 24:4620–5.
3. Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009; 27:5124–30.

4. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005; 23:5644–50.

5. You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020; 63:1191–222.

6. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28:iv22–40.

7. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:874–901.

8. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006; 93:1215–23.

9. Erlandsson J, Holm T, Pettersson D, Berglund A, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017; 18:336–46.

10. Kairevice L, Latkauskas T, Tamelis A, Petrauskas A, Pauzas H, Zvirblis T, et al. Preoperative long-course chemoradiotherapy plus adjuvant chemotherapy versus short-course radiotherapy without adjuvant chemotherapy both with delayed surgery for stage II–III resectable rectal cancer: 5-Year survival data of a randomized controlled trial. Medicina (Kaunas). 2017; 53:150–8.
11. Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012; 30:3827–33.

12. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006; 355:1114–23.
13. Boulis-Wassif S, Gerard A, Loygue J, Camelot D, Buyse M, Duez N. Final results of a randomized trial on the treatment of rectal cancer with preoperative radiotherapy alone or in combination with 5-fluorouracil, followed by radical surgery. Trial of the European Organization on Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. Cancer. 1984; 53:1811–8.
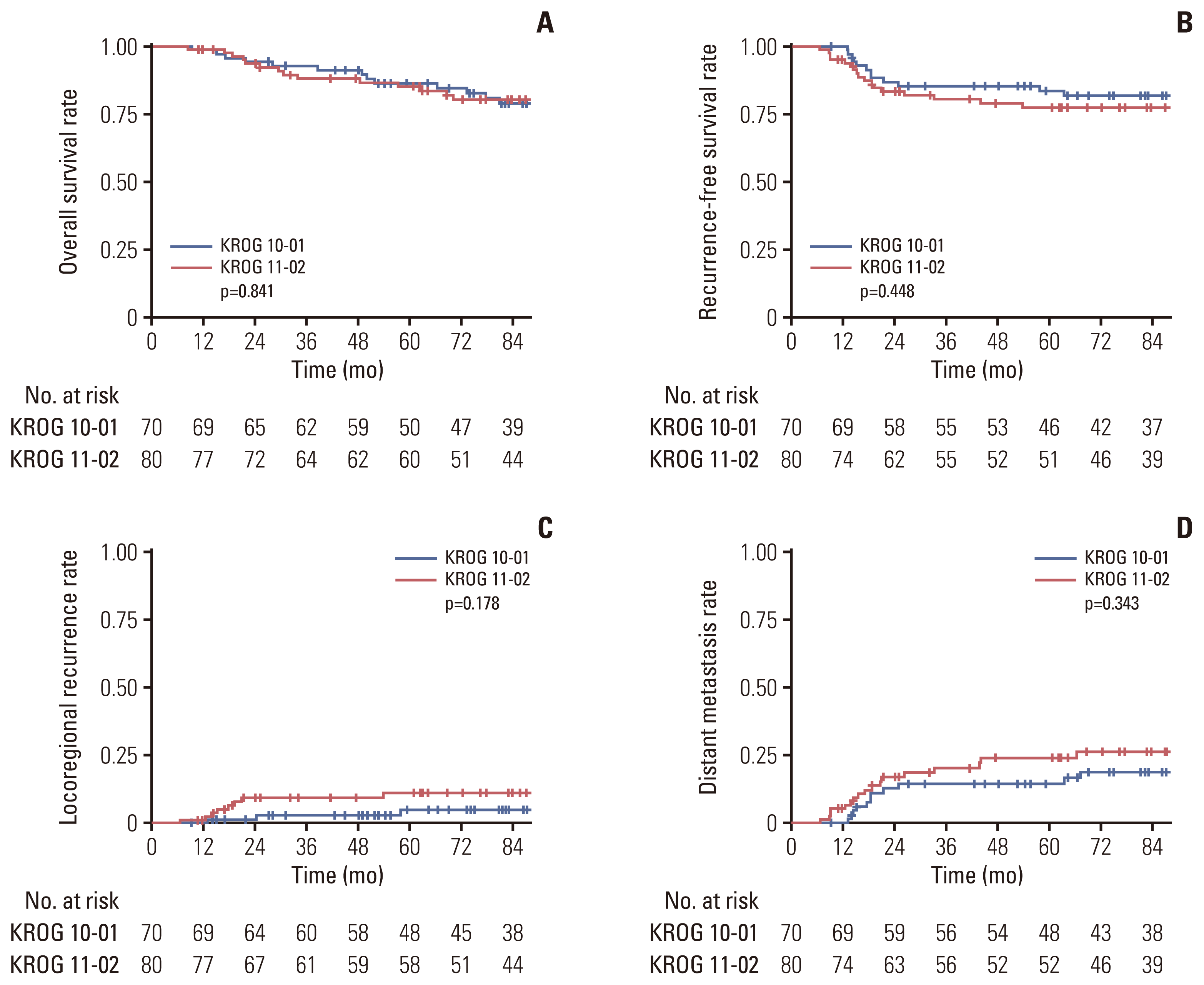
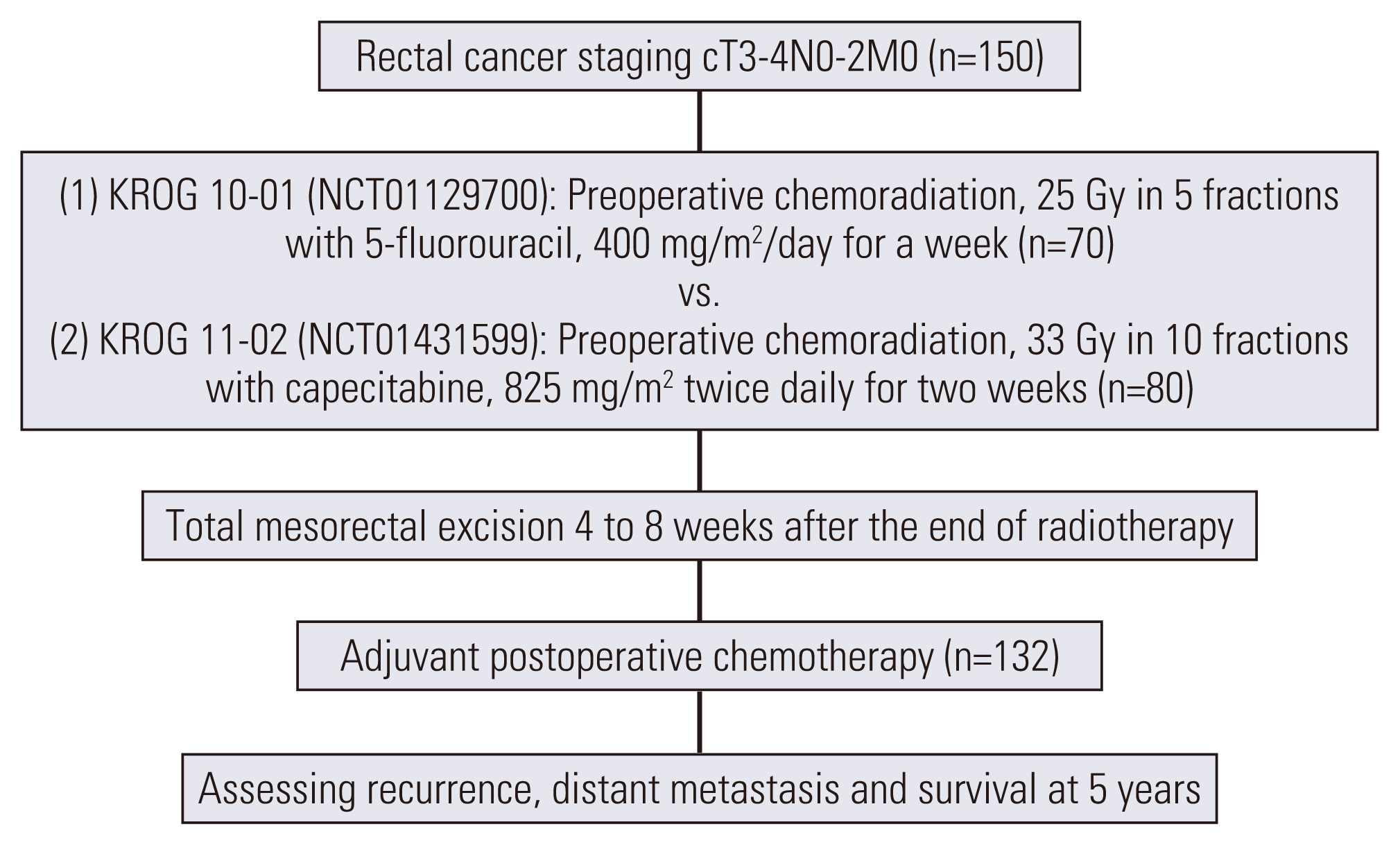
14. Yeo SG, Oh JH, Kim DY, Baek JY, Kim SY, Park JW, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01). Int J Radiat Oncol Biol Phys. 2013; 86:34–9.

15. Lee JH, Kim JG, Oh ST, Lee MA, Chun HG, Kim DY, et al. Two-week course of preoperative chemoradiotherapy followed by delayed surgery for rectal cancer: a phase II multi-institutional clinical trial (KROG 11-02). Radiother Oncol. 2014; 110:150–4.
16. Koeck J, Kromer K, Lohr F, Baack T, Siebenlist K, Mai S, et al. Small bowel protection in IMRT for rectal cancer: a dosimetric study on supine vs. prone position. Strahlenther Onkol. 2017; 193:578–88.
17. White R, Foroudi F, Sia J, Marr MA, Lim Joon D. Reduced dose to small bowel with the prone position and a belly board versus the supine position in neoadjuvant 3D conformal radiotherapy for rectal adenocarcinoma. J Med Radiat Sci. 2017; 64:120–4.

18. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.

19. Wagman R, Minsky BD, Cohen AM, Guillem JG, Paty PP. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys. 1998; 42:51–7.

20. Wang X, Zheng B, Lu X, Bai R, Feng L, Wang Q, et al. Preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer: meta-analysis with trial sequential analysis of long-term survival data. PLoS One. 2018; 13:e0200142.
21. Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, et al. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg. 2017; 265:882–8.
22. Liu SX, Zhou ZR, Chen LX, Yang YJ, Hu ZD, Zhang TS. Short-course versus long-course preoperative radiotherapy plus delayed surgery in the treatment of rectal cancer: a meta-analysis. Asian Pac J Cancer Prev. 2015; 16:5755–62.

23. Zhou ZR, Liu SX, Zhang TS, Chen LX, Xia J, Hu ZD, et al. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2014; 23:211–21.

24. Dapper H, Rodriguez I, Munch S, Peeken JC, Borm K, Combs SE, et al. Impact of VMAT-IMRT compared to 3D conformal radiotherapy on anal sphincter dose distribution in neoadjuvant chemoradiation of rectal cancer. Radiat Oncol. 2018; 13:237.

25. Reddy AV, Hill CS, Sehgal S, Ding D, Hacker-Prietz A, He J, et al. Impact of somatic mutations on clinical and pathologic outcomes in borderline resectable and locally advanced pancreatic cancer treated with neoadjuvant chemotherapy and stereotactic body radiotherapy followed by surgical resection. Radiat Oncol J. 2021; 39:304–14.

26. Chan AK, Wong AO, Jenken DA. Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer--is it equivalent to 5-FU infusion plus leucovorin and radiotherapy? Int J Radiat Oncol Biol Phys. 2010; 76:1413–9.

27. Desai SP, El-Rayes BF, Ben-Josef E, Greenson JK, Knol JA, Huang EH, et al. A phase II study of preoperative capecitabine and radiation therapy in patients with rectal cancer. Am J Clin Oncol. 2007; 30:340–5.

28. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012; 13:579–88.

29. Song JH, Jeong JU, Lee JH, Kim SH, Cho HM, Um JW, et al. Preoperative chemoradiotherapy versus postoperative chemoradiotherapy for stage II–III resectable rectal cancer: a meta-analysis of randomized controlled trials. Radiat Oncol J. 2017; 35:198–207.

30. Ngan SY, Michael M, Mackay J, McKendrick J, Leong T, Lim Joon D, et al. A phase I trial of preoperative radiotherapy and capecitabine for locally advanced, potentially resectable rectal cancer. Br J Cancer. 2004; 91:1019–24.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download