This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Aquaporin (AQP) expression has been investigated in various malignant neoplasms, and the overexpression of AQP is related to poor prognosis in some malignancies. However, the expression of AQP protein in clear cell renal cell carcinoma (ccRCC) has not been extensively investigated by immunohistochemistry with large sample size.
Methods
We evaluated the AQP expression in 827 ccRCC with immunohistochemical staining in tissue microarray blocks and classified the cases into two categories, high and low expression.
Results
High expression of aquaporin-1 (AQP1) was found in 320 cases (38.7%), but aquaporin-3 was not expressed in ccRCC. High AQP1 expression was significantly related to younger age, low TNM stage, low World Health Organization/International Society of Urologic Pathology nuclear grade, and absence of distant metastasis. Furthermore, high AQP1 expression was also significantly associated with longer overall survival (OS; p<.001) and progression-specific survival (PFS; p<.001) and was an independent predictor of OS and PFS in ccRCC.
Conclusions
Our study revealed the prognostic significance of AQP1 protein expression in ccRCC. These findings could be applied to predict the prognosis of ccRCC.
Keywords: Aquaporin, Clear cell renal cell carcinoma, Immunohistochemistry, Prognosis
Renal cell carcinoma (RCC) is one of the most common and clinically important malignant neoplasms of the urinary tract. Its prognosis is favorable when found early and completely resected, but it could be potentially aggressive and fatal with advanced stage, high World Health Organization (WHO)/International Society of Urologic Pathology (ISUP) nuclear grade, and/or distant metastasis. Clear cell renal cell carcinoma (ccRCC) is the most common histologic subtype of RCC and is known to have an unfavorable prognosis compared with other common subtypes. Therefore, ccRCC has been the main topic in several genitourinary oncologic and pathologic studies.
Aquaporins (AQPs) are cell membrane proteins that act as water transporters, facilitating the permeation of water through the plasma membrane [
1]. Twelve members of the AQP family are expressed in different types of normal tissue and cells. Earlier studies have shown that AQPs are associated with several characteristics of malignancies, such as cellular motility, migration, angiogenesis, and metastasis, and may be potential targets for anticancer therapies [
2-
4]. The overexpression of AQP has been observed in several malignancies. For instance, Kang et al. [
5] investigated the relationship between the levels of AQP1, AQP3, and AQP5 expression by immunohistochemistry and several clinical variables in colorectal carcinoma and concluded that lymph node metastasis is positively related to a high level of AQP expression. In a systematic review regarding the relationship of AQP1, AQP3, and AQP5 expressions with different tumors reported by Moosavi and Elham [
6], overexpression of AQPs contributed to the pathogenesis of malignant neoplasms and was associated with unfavorable clinical outcomes in malignancies, e.g., gastric, breast, pancreatic, lung, and colorectal carcinomas.
Notably, AQPs may have an unusual relationship with tumorigenesis in genitourinary tumors compared to malignancies of other origins. This may be explained by the expression of AQPs in the normal renal parenchyma and urothelium. Ticozzi-Valerio et al. [
7] showed that the proteomic level of AQP1 expression was significantly lower in ccRCC cells than in adjacent normal proximal tubular epithelial cells using electrophoresis and immunoblotting in small number of ccRCC specimen. Huang et al. [
8] reported that the high AQP1 mRNA expression is related to less aggressive characteristics, such as lower grade, smaller tumor size, lower pathological stage, absence of microvascular invasion and symptomatic disease, as well as more favorable outcomes, such as better cancer-specific and cancer-free survival using quantitative real time polymerase chain reaction in ccRCC. Otto et al. [
9] demonstrated that the loss of AQP3 expression by immunohistochemistry is significantly associated with worse prognosis-free survival in non-muscle invasive (stage pT1) urothelial carcinoma of the urinary bladder. Therefore, one could hypothesize that AQP expression is related to the degree of aggressiveness in urinary tract neoplasms. However, there has not yet been a study that evaluated the AQP protein expression in ccRCC by immunohistochemistry in a large number of samples.
In this study, we conducted immunohistochemical staining of AQP1 and AQP3 in ccRCC tissue microarrays (TMAs) from nephrectomy specimens and evaluated the expression of AQP and its clinical significance.
MATERIALS AND METHODS
The subjects of this study were 827 primary sporadic ccRCC patients who underwent partial or radical nephrectomy from 2006 to 2011 at Seoul National University Hospital. The clinical data corresponding to tissue specimens were collected from electronic medical records of the hospital. Clinical stage and nuclear grade of carcinomas were assigned according to the American Joint of Committee on Cancer 8th TNM staging and World Health Organization/International Society of Urologic Pathology (WHO/ISUP) nuclear grade, respectively. The representative tumor portions of ccRCC specimen blocks were selected in each case. Two representative cores with 2 mm-diameter was taken from tumor blocks and embedded to new recipient blocks using trephine apparatus (Superbiochips Laboratories, Seoul, Korea).
Tissue microarray (TMA) blocks were cut to make several unstained slides for histopathological and immunohistochemical analyses. Hematoxylin and eosin slides were made for histopathological assessment of TMAs. Immunohistochemical staining was performed using Ventana Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ, USA). The 4-µm-thick slides were stained with anti-AQP1 mouse monoclonal IgG1 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-AQP3 IgG1 antibody (1:100, Abcam, Cambridge, UK). The intensity of AQP expression in each slide was scored semi-quantitatively, incorporating the intensity and percentage of RCC cells with membranous stain positivity. The intensity was evaluated with scores of 0–3: 0 for negative staining; 1 for faint positivity; 2 for weak to moderate positivity; and 3 for strong positivity. The percentage of positive tumor cells was scored on a 0–4-point basis: 0 for 0%; 1 for 1 to 25%; 2 for 26 to 50%; 3 for 51–75%; and 4 for more than 75%. Next, the total AQP expression score was produced by the sum of two points and allocated to three categories: AQP-negative for 0–2; weakly AQP-positive for 3–5; and strongly AQP-positive for 6–7 [
5]. Finally, both AQP-negative and weakly AQP-positive categories were considered as losing AQP1 expression significantly and allocated to “low” AQP1 expression group, and strongly AQP-positive category was allocated to “high” AQP1 expression group.
Clinical data and immunohistochemical AQP expression scores of TMAs were integrated and statistically analyzed using the R programming language (ver. 4.2.2) with the packages ‘survival’, ‘survminer’ and ‘dplyr’. The overall survival (OS) duration was defined as the interval from the date of surgical resection to death or the last follow-up. The progression-free survival (PFS) duration was defined as the interval from the date of surgical resection to the event of progression, such as death; local recurrence; distant metastasis; or disease progression after chemotherapy, immunotherapy, or radiotherapy. Kaplan-Meier analysis and log-rank tests were conducted to evaluate and compare OS and PFS between patients divided by binominal or dichotomized continuous clinicopathological variables, i.e., low (1, 2) and high (3, 4) TNM stage and/or WHO/ISUP nuclear grade, as well as with weakly and strongly AQP-positive ccRCC. Multivariate Cox regression model was established by statistically significant variables in univariate analyses, and the multivariate analysis was applied to assess the potential clinical significance of AQP expression by immunohistochemistry. A p-value less than .05 was interpreted as statistically significant.
RESULTS
Patient characteristics
Clinicopathological characteristics of the patients are shown in
Table 1. The group showed prominent male predominance, and the mean age was 56.5 years. The average size of the tumor was 4.2 cm. TNM stage 1 was the most common of the four stages. The WHO/ISUP nuclear grades 2 and 3 accounted for major proportion in the group. The mean OS interval and PFS interval were 91.8 and 79.5 months, respectively.
Aquaporin expression in ccRCC
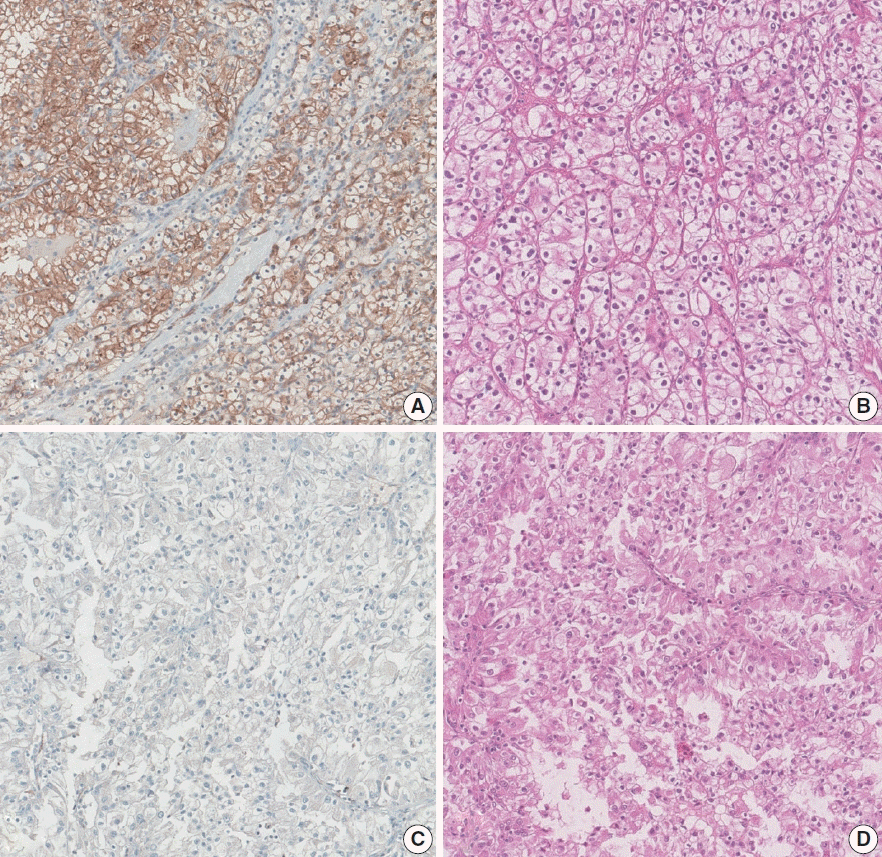
AQP immunohistochemical staining was mainly present in the membrane. After evaluation, AQP1 expression was high in 320 cases (38.7%), and low in 507 cases (61.3%) (
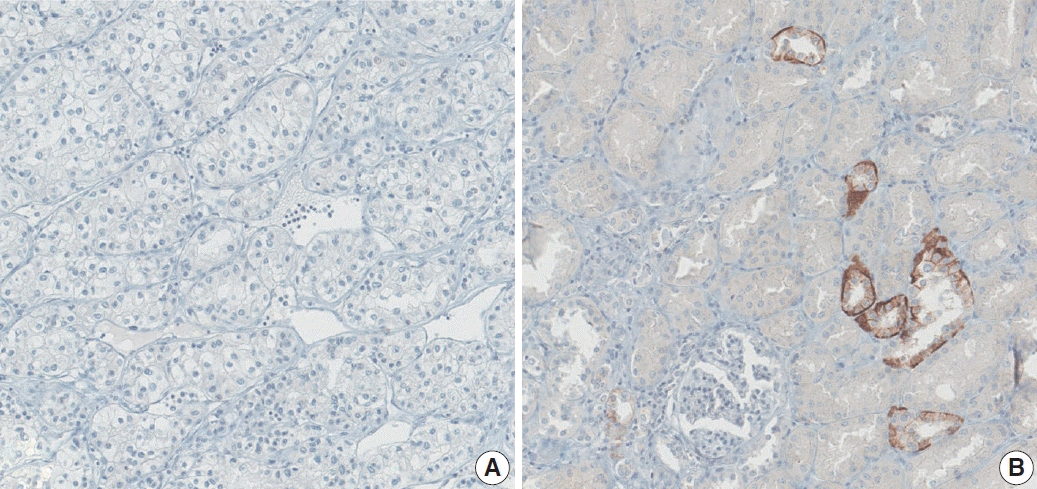
Fig. 1). On the other hand, the AQP3 expression level was exceptionally low in all cases compared to AQP1 expression, preventing it from being investigated statistically (
Fig. 2).
Relationships of aquaporin expression and clinicopathological characteristics
Next, we examined whether there were statistically significant correlations between clinicopathological characteristics and AQP1 expression levels. First, we assigned the patient population to each of the four characteristics. The cutoff for patient age was 55 years. TNM stage and nuclear grade were grouped as “low” for stage/grade 1 to 2 and “high” for stage/grade 3 to 4. The state of distant metastasis (M category) was incorporated for the analysis. Consequently, Fisher’s exact test demonstrated that lower AQP1 expression presented statistically significant correlation with higher (55 or more) age of the patient, “high” TNM stage (3 or 4) and nuclear grade (3 or 4), the presence of distant metastasis (M1), and presence of microvascular invasion (
Table 2).
Impact of aquaporin expressions on survival
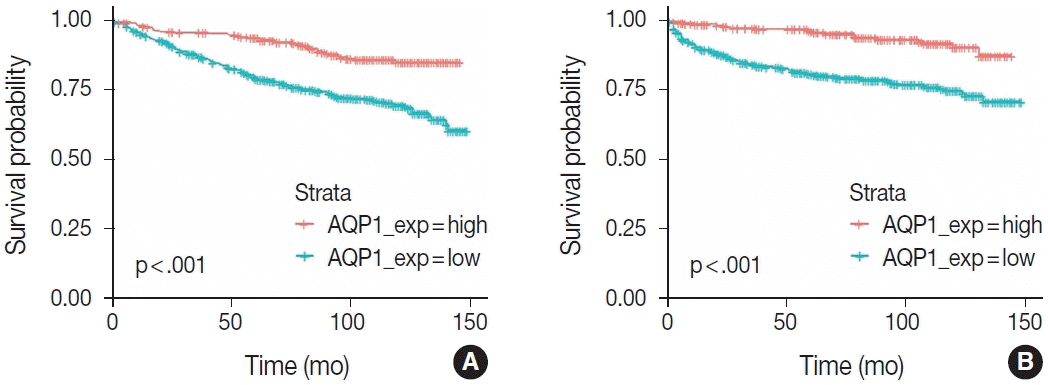
Next, we investigated the impact of AQP1 expression level on OS and PFS periods. The survival curves corresponding to OS and PFS, derived from the Kaplan-Meier method are shown in
Fig. 3. Survival rates according to AQP1 expression level demonstrated significant difference in both curves, inferring that decreased expression level of AQP1 in clear cell RCC is associated with undesirable clinical outcomes and shorter survival periods. This finding is in accordance with prior statistical analyses showing the relationship of low AQP1 expression level with worse clinicopathological features.
We also conducted multivariate Cox proportional hazards regression analysis corresponding to both OS and PFS to investigate the ability of AQP1 expression to be an independent risk factor in ccRCC. First, univariate analyses were conducted for several clinicopathological factors (
Table 3). In univariate analyses, TNM stage, WHO/ISUP nuclear grade, and AQP1 expression level were selected as basic components of the multivariate Cox proportional hazards model (
Tables 3,
4). As a result, lower AQP1 expression level showed statistical significance to be an independent predictor of worse OS and PFS in the model.
DISCUSSION
In this study, we demonstrated that lower expression of AQP1 is related to more advanced clinical stage, higher WHO/ISUP nuclear grade, microvascular invasion, and shorter OS and PFS. To our knowledge, this study provides the first evidence that AQP expression level as evaluated by immunohistochemistry has a similar relationship to ccRCC, compared with prior proteomic [
7] and transcriptomic [
8] studies of AQP1.
AQPs are expressed in normal kidney parenchyma, especially in tubules and the collecting duct system [
10]. AQP1 is known to be differentially expressed in proximal tubular epithelial cells, whereas AQP2, AQP3, and AQP4 are mainly located in distal tubules and collecting ducts. Considering that ccRCCs arise from proximal tubular epithelial cells and that a higher WHO/ISUP nuclear grade is significantly associated with a low AQP1 expression level in ccRCC, we can speculate that the loss of AQP1 in ccRCC reflects the loss of differentiation, which is commonly observed in carcinogenesis. As stated in previous publications covering non-urogenital malignancies, aberrant expression of AQPs could occur [
5,
11] and may have different correlations with RCCs. In this study, AQP3 was the candidate for such aberrant expression in ccRCC, not showing evidence of expression by immunohistochemistry. This point is worth investigating in additional studies.
Some previous studies showed that higher AQP1 expression is associated with aggressive clinicopathological characteristics in some malignancies, such as pancreatic ductal adenocarcinoma [
12] and cholangiocarcinoma [
13]. On the contrary, the results of this study showed that lower AQP1 expression is associated with aggressive clinicopathological characteristics in ccRCC. The mechanisms for the opposite effect in different carcinomas are not well investigated and additional studies are needed to demonstrate the underlying mechanism of this difference.
Further research on the correlation between AQPs and other common and clinically significant subtypes of RCC, e.g., papillary and chromophobe RCC, is anticipated. Other subtypes of AQP might show different relationships between aggressive clinicopathological parameters of RCC. Xu et al. [
14] reported elevated AQP9 mRNA expression in ccRCCs of advanced clinical stages with shorter OS and PFS, suggesting that AQP9 could act as an oncogene in ccRCC. This could be a possible topic for later studies about AQPs and malignancies.
Several previous studies have utilized interventional agents to investigate the potential of AQP as a target of anticancer therapies. AqB013, a small molecule AQP1 inhibitor, demonstrated the potential to prevent cancer cell migration and angiogenesis in colorectal carcinoma cell lines [
15]. The microRNA miR-874 showed the ability to inhibit AQP3 gene transcription and downregulate the level of AQP3 protein expression, impeding gastric carcinoma cell lines to form tumors, migrate, and metastasize [
16]. These results propose the possibility that loss of AQP expression in ccRCC could be an exploitable characteristic to be utilized for targeted anticancer therapies.
The study may have some potential limitations, which might have impacted the results and are worth discussing. For instance, only ccRCC cases were incorporated in the study, leaving other common or clinically important subtypes of renal cell carcinomas uninvestigated, such as papillary RCC, chromophobe RCC, collecting duct carcinoma, and medullary carcinoma. Further research is required to investigate the association of AQP expression and these renal malignancies. Despite this, we demonstrated the potential utility of aquaporin family proteins in ccRCC as tools to predict the clinical behavior and prognosis of this clinically important malignancy.
In conclusion, loss of AQP1 expression, evaluated by immunohistochemistry, is related to worse clinicopathological parameters and shorter survival in ccRCC. It would be potentially applied to predict prognosis and develop target anticancer agents.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download