1. Lee AH, Hodi Z, Soomro I, et al. Histological clues to the diagnosis of metastasis to the breast from extramammary malignancies. Histopathology. 2020; 77:303–13.
2. Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017; 11:279–89.
3. Nathan P, Cohen V, Coupland S, et al. Uveal melanoma UK national guidelines. Eur J Cancer. 2015; 51:2404–12.
4. Sato T. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol. 2010; 37:127–38.
5. Taran-Munteanu L, Hartkopf A, Eigentler TK, Vogel U, Brucker S, Taran FA. A case of choroidal melanoma metastatic to the breast. Geburtshilfe Frauenheilkd. 2016; 76:579–81.
6. DeLair DF, Corben AD, Catalano JP, Vallejo CE, Brogi E, Tan LK. Non-mammary metastases to the breast and axilla: a study of 85 cases. Mod Pathol. 2013; 26:343–9.
7. Drueppel D, Schultheis B, Solass W, Ergonenc H, Tempfer CB. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015; 35:1709–13.
8. Zhou P, Chang N, Abraham SC, et al. Metastatic nonhematopoietic neoplasms to the breast: a study of 238 cases. Hum Pathol. 2022; 125:59–67.
9. Chopra JS, Chandar K. Bilateral breast metastases from malignant melanoma of the eye. Aust N Z J Surg. 1972; 42:183–5.
10. Demirci H, Shields CL, Shields JA, Eagle RC Jr, Honavar SG. Bilateral breast metastases from choroidal melanoma. Am J Ophthalmol. 2001; 131:521–3.
11. Esposito A, Gentile D, Ghidotti I, Allegri M. A rare case of metastasis of choroidal melanoma in male breast: mammographic and ultrasonographic diagnosis. Radiol Med. 1994; 88:487–8.
12. McCormick A, Rennie I. Bilateral breast metastases from choroidal melanoma. Am J Ophthalmol. 2001; 132:951–2.
13. McCarthy C, Kalirai H, Lake SL, Dodson A, Damato BE, Coupland SE. Insights into genetic alterations of liver metastases from uveal melanoma. Pigment Cell Melanoma Res. 2016; 29:60–7.




 PDF
PDF Citation
Citation Print
Print



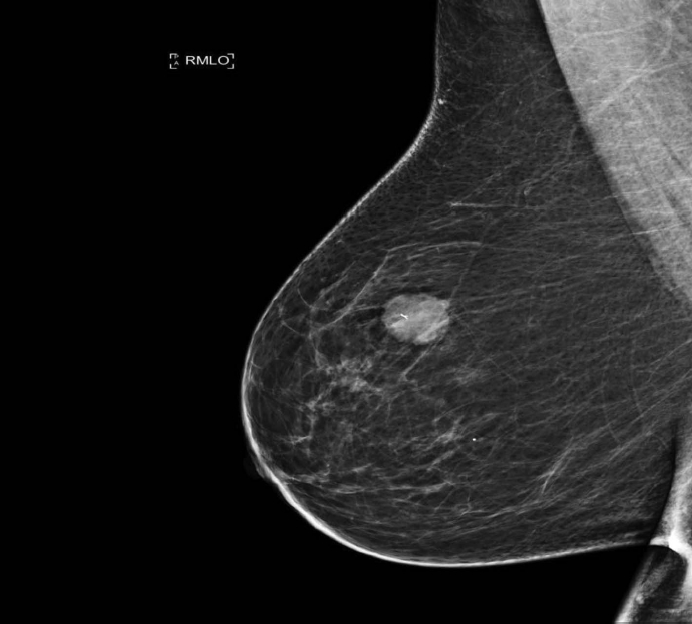
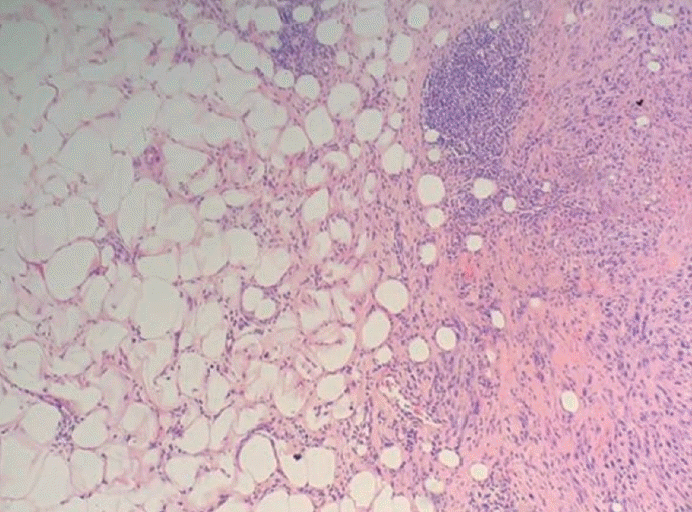
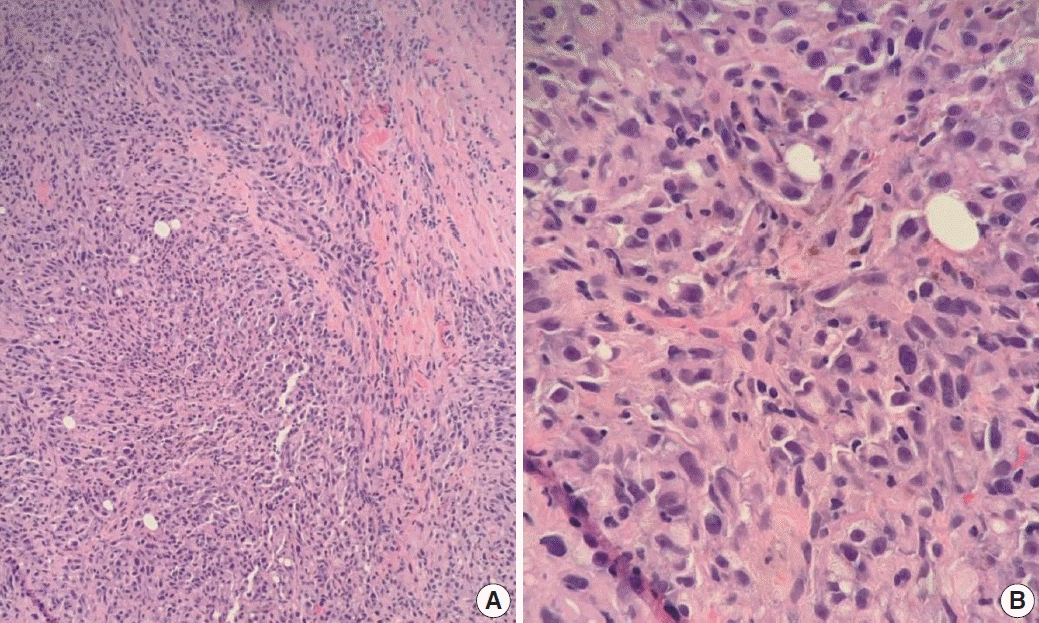
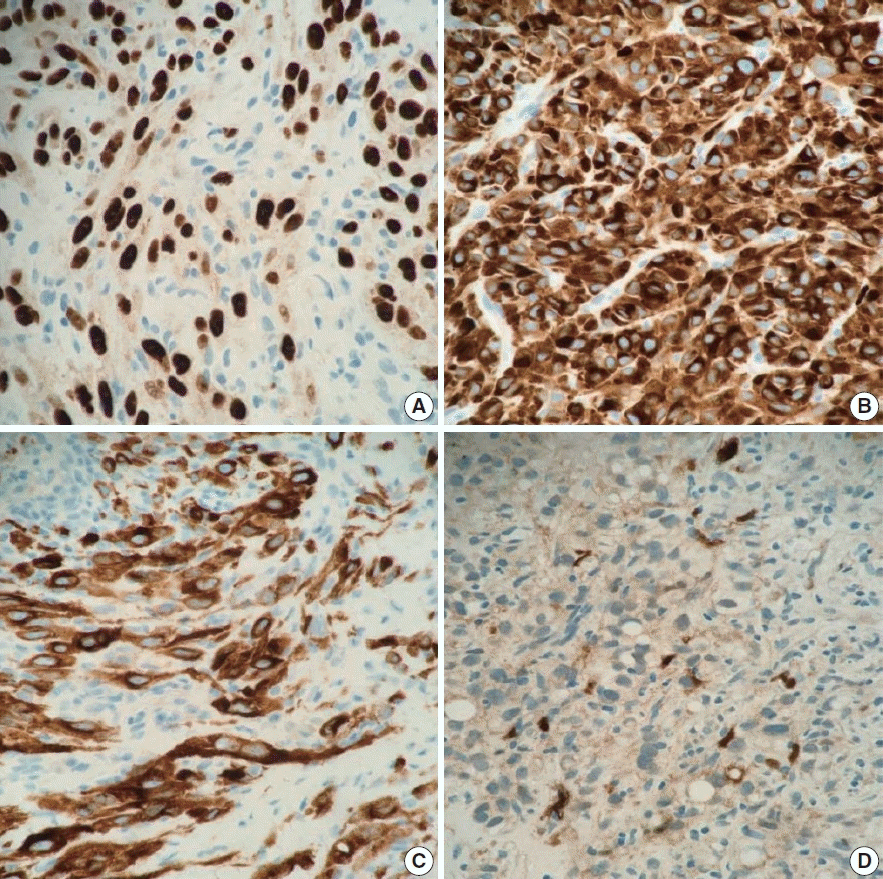
 XML Download
XML Download