Abstract
Objective
Vaginal morphology and pelvic floor muscle (PFM) strength may influence sexual stimulation, sensation, and orgasmic response. This study aimed to determine the relationship between female sexual function and PFM strength and vaginal morphology (represented by vaginal resting tone and vaginal volume) in women with stress urinary incontinence (SUI).
Methods
Forty-two subjects with SUI were recruited for the study. Female sexual function was measured using the female sexual function index (FSFI) questionnaire. PFM strength was measured by digital palpation. Vaginal resting tone (mmHg) and vaginal volume (mL) were measured using a perineometer. The significance of the correlations between female sexual function and PFM function and hip muscle strength was assessed using Pearson’s correlation coefficients. If a significant correlation between vaginal morphology and FSFI score was confirmed using Pearson’s correlation, the cutoff value was confirmed through a decision tree.
Results
PFM strength was significantly correlated with desire (r=0.397), arousal (r=0.388), satisfaction (r=0.326), and total (r=0.315) FSFI scores. Vaginal resting tone (r=-0.432) and vaginal volume (r=0.332) were significantly correlated with the FSFI pain score. The cutoff point of vaginal resting tone for the presence of pain-related sexual dysfunction was >15.2 mmHg.
Vaginal size, caliber, and tone may affect sexual stimulation, sensation, and orgasmic response [1]. Trauma to the vagina and pelvic floor muscles (PFMs) because of aging, pregnancy, and vaginal childbirth may contribute to vaginal laxity and PFM dysfunction, resulting in reduced sexual quality of life and loss of physical and sexual sensation during intercourse [2-4].
Non-invasive treatment to strengthen PFM contractions includes PFM training with or without electrical stimulation of the vagina and is the primary recommended treatment for stress urinary incontinence (SUI) [5]. Although surgery can be performed to tighten the introitus, pain at the incision site can lead to dyspareunia after the procedure. Moreover, pelvic reconstructive surgery may decrease the vaginal length and introital diameter [6,7], negatively affecting female sexual function and increasing the risk of dyspareunia.
The PFMs contribute to female sexual function, such as the grip experienced by a partner and the degree of sensation experienced by women during vaginal intercourse [8]. Orgasm is defined as involuntary rhythmic PFM contractions [9]. PFM dysfunction has been categorized as normal, high tone (overactive), low tone (underactive), and non-functioning [10,11]. Overactive PFMs require relaxation of muscle tension. In contrast, low-tone PFMs that cannot voluntarily contract are typically categorized as underactive. “Non-functioning” refers to the absence of PFM activity. These clinically varying states of PFM tone affect female sexual function.
If vaginal morphological factors, such as vaginal tone and volume, are excessively high or low, they may cause female sexual dysfunction. Thus, it is necessary to determine the optimal vaginal tone and volume for female sexual function. In addition, because both vaginal morphological factors and PFM activity affect female sexual function, it is necessary to demonstrate the relationship between these variables.
Thus, this study aimed to determine the relationship between sexual function and vaginal morphology and PFM strength in women with SUI. The study also aimed to establish the cutoff value for predicting the risk of female sexual dysfunction using the decision tree method.
Before participating in the study, we explained all experimental procedures to the subjects, and they provided written informed consent. The study was approved by our institutional review board. In total, 42 women with SUI participated in the present study. We included women 1) aged 30-60 years, 2) reporting leakage episodes more than once a week, and 3) diagnosed with SUI by a urogynecologist. Subjects were excluded if they 1) did not meet the inclusion criteria, 2) were pregnant/planning to become pregnant, 3) had a history of pelvic or abdominal surgery within the last 6 months, and 4) had a neurological disease. The current study was conducted between September and December 2018 at an obstetrics and gynecology clinic in Seoul, South Korea.
An a priori power analysis of pilot data was performed using G*power (version 3.1.3; University of Trier, Trier, Germany) to calculate the necessary sample size. A power of 0.80, α level of 0.05, and correlation ρ of 0.40 were considered sufficient to identify a significant relationship between the female sexual function index (FSFI) pain domain score and vaginal volume and resting tone [12-14]. At least 37 subjects were required to detect a significant relationship between female sexual function and vaginal morphology.
Female sexual function was measured using the Korean version of the validated FSFI [15,16]. The FSFI is a self-administered questionnaire that consists of 19 items comprising six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. Each domain is scored from 0 or 1 to 6 points, and the total FSFI score is a sum of the six domain scores (2-36 points), with higher scores indicating better female sexual function [15].
Digital palpation is one of the most common clinical methods for measuring PFM strength and a woman’s ability to contract the PFMs effectively [17,18]. Subjects were asked to contract their PFM in hook-lying position, and digital palpation was used for subjective assessment. The examiner’s index finger was inserted approximately 4 cm into the vagina [19]. PFM strength was assessed by digital palpation using a modified Oxford muscle grading scale (0: no contraction; 1: minor muscle contraction-‘flicker’; 2: weak muscle contraction; 3: moderate muscle contraction; 4: good muscle contraction; 5: strong muscle contraction against resistance) [19].
Vaginal resting tone and volume were measured with a VVP3000 perineometer (QLMED Ltd, Seongnam, Korea) in the hook-lying position for all subjects. The vaginal probe of the perineometer (diameter, 24 mm; total length, 115 mm; and active vaginal length, 66 mm) was linked to a pressure transmitter with a microprocessor with latex tubing [14]. The vaginal resting tone was defined as the initial pressure (mmHg) of the compressed vaginal wall in the resting position. After the vaginal probe was inserted, subjects were asked to relax their PFMs and abdominal and hip muscles and breathe quietly. After measuring the vaginal resting tone, the vaginal volume was measured. Vaginal volume is defined as the amount of air injected into the vaginal probe until 60 mmHg pressure is reached. Vaginal volume was measured twice, and the mean injected air volume was recorded in millimeters.
The Kolmogorov-Smirnov Z-test was applied to identify the normal distribution of variables. Pearson’s correlation coefficients were calculated to identify the correlations between FSFI scores and vaginal morphology and PFM strength. A correlation coefficient (r) of >0.75 was considered “good to excellent”, 0.50-0.75 was “moderate to good”, 0.25-0.50 was “fair”, and 0.00-0.25 was “little or no” relationship [20]. For secondary analysis, multiple regression models using stepwise selection included age, body mass index, duration of symptoms, PFM strength, vaginal volume, and vaginal resting tone as independent variables, with FSFI domains as dependent variables. The coefficient of determination (R2) showed the explanatory power of the variables. If a statistically significant correlation between vaginal morphology and FSFI was confirmed using Pearson’s correlation, the cutoff value was determined with a decision tree. A decision tree analysis was used to identify factors that predicted sexual dysfunction. The conditions of the decision tree analysis were as follows: maximization of the decrease in impurity (minimization of the Gini index); minimum value of the parent node, 10; and minimum value of the child node, 5. All statistical analyses were performed using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL, USA) with alpha set at 0.05.
Table 1 shows the characteristics of the 42 participants recruited in our study. Table 2 displays the average, standard deviation, and range of all variables. Table 3 shows the correlations of the six FSFI domain scores and the total FSFI score with PFM strength, vaginal resting tone, and vaginal volume. Significant correlations were confirmed between desire (r=0.397), arousal (r=0.388), satisfaction (r=0.326), and total (r=0.315) FSFI scores and PFM strength. Significant correlations were confirmed between the FSFI pain score and vaginal resting tone (r=-0.432) and vaginal volume (r=0.332).
In a stepwise regression model, age and PFM strength accounted for 20.1% of the FSFI-desire score (Table 4). The PFM strength accounted for 11.9% and 10.8% of the FSFI-arousal and FSFI-satisfaction scores, respectively (Table 4). The vaginal resting tone accounted for 18.7% of the FSFI-pain score (Fig. 1 and Table 4). However, in the FSFI total score, only age was entered into the model and accounted for 12.3% of the score. Post hoc power analyses using G*power were calculated by setting the significance level P=0.05, total sample size=42, number of predictors=6, and effect size f2=0.404-0.628 (by calculating from squared multiple correlation=0.288-0.386) [21]. The power value was computed to be 0.820-0.961. Thus, the post hoc power analysis confirmed that the power (1-β error probability) was sufficient for multiple stepwise regression.
Because correlations between the FSFI pain score and vaginal resting tone and volume were confirmed, we aimed to determine which variables explained the FSFI pain scores through the decision tree and to confirm the cutoff value (Fig. 2). Subjects with and without pain-related sexual dysfunction were identified based on an FSFI pain score of 2.5 (pain score <2.5 [n=11] vs. pain score ≥2.5 [n=31]) [22]. To classify the presence of sexual difficulty in each domain, a score of 40% or less of the maximum value of the pain domain (<2.5) was selected as the pain score cutoff [22]. A classification decision tree to identify the presence of pain-related sexual dysfunction based on vaginal morphology (vaginal resting tone and volume) was derived. Fig. 1 shows the classification tree for pain-related sexual dysfunction, which had two terminal nodes. The classification tree showed vaginal resting tone as the first predictor of pain-related sexual dysfunction. The cutoff point of vaginal resting tone for the presence of pain-related sexual dysfunction was >15.2 mmHg. The accuracy of the classification tree model was 78.6%.
As vaginal morphology may be related to sexual function and dyspareunia, we confirmed the relationship between vaginal morphology and the pain score in FSFI. However, no significant correlations were identified between vaginal morphology and other FSFI subscores. PFM strength was significantly correlated with more female sexual functions (desire, arousal, satisfaction, and total FSFI scores). Although the significant correlations between variables were moderate to good, psychosocial factors and numerous other factors, including age, obesity, menopausal status, educational level, income, interactions with a partner, and the physical health status of the women could also contribute to female sexual function [23,24]. Because various variables contribute to female sexual function independently or in combination, further study is needed on the influencing factors, including physical, psychosocial, and environmental factors, on female sexual function. Vaginal rejuvenation surgery is often considered for enhancing vaginal tone and sexual friction in women with a wide vagina or vaginal laxity due to aging or vaginal delivery [25]. However, because of the relationship between vaginal morphology and the FSFI pain score, surgical decisions regarding vaginal rejuvenation should be made carefully. Moreover, more studies on the optimal vaginal tone and volume for female sexual function need to be performed to provide guidelines for vaginal rejuvenation surgery.
The present study confirmed significant correlations between the desire (r=0.397), arousal (r=0.388), satisfaction (r=0.326), and total (r=0.315) FSFI scores and PFM strength. Also, in multiple stepwise regression, PFM strength was entered in models for the desire, arousal, and satisfaction domains. Although it is difficult to directly compare our study with previous studies because of differences in measurements of PFM strength and female sexual function, a previous study showed similar significant correlations between PFM strength and the behavioral/emotive domain score (r=0.441), physical domain score (r=0.322), and the overall total score (r=0.387) of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ) [13]. Various physiological mechanisms have been postulated by which PFM strength could influence female sexual function. First, PFM weakness could affect the inability to achieve orgasm, defined as involuntary rhythmic muscle contraction [26]. Second, PFM weakness could imply insufficient contractions for arousal and vaginal friction [27]. Better arousal, desire, and satisfaction indicate greater PFM strength in women with SUI [28]. Third, PFM contraction could increase blood flow to the clitoris [29] and enhance clitoral sensitivity [30]. Thus, our results could also be considered to improve female sexual function through PFM strengthening or training in women with SUI.
Concerning the relationship between vaginal morphology and female sexual function, we confirmed significant correlations between the FSFI pain score and vaginal resting tone (r=-0.432) and vaginal volume (r=0.332). Also, in multiple stepwise regression, vaginal resting tone was entered into models for pain domain. Generally, vaginal size is measured by sonography, and a large epidemiological study of gynecological patients confirmed a mean total vaginal length of 9.6 cm and a mean genital hiatus of 2.9 cm [31]. However, the relationship between these measurements and sexual function is controversial. The size of the genital hiatus did not significantly differ between women with and without sexual dysfunction (FSFI total score <26) [32], and total vaginal length was weakly correlated with FSFI total score (r=0.122, P=0.027) [32]. In contrast, pelvic reconstructive surgery decreases the vaginal length and genital hiatus size, which could negatively influence female sexual function and increase dyspareunia [6,7].
Across 14 studies, the mean preoperative PISQ-12 score was not related to the mean preoperative total vaginal length. The mean postoperative PISQ-12 was also not related to preoperative total vaginal length, postoperative total vaginal length, or change in total vaginal length [33]. Across nine studies, neither pre- nor postoperative genital hiatus was related to PISQ-12 [33]. Although it is difficult to make direct comparisons with previous studies because vaginal morphology and female sexual function are assessed differently, our results indicate that increased vaginal resting tone and decreased vaginal volume are associated with worse pain in female sexual function. Thus, vaginal resting tone and vaginal volume need to be considered as risk factors of dyspareunia.
Excessively high or low vaginal resting tone or volume could cause female sexual dysfunction. A decision tree analysis was performed to confirm factors that predicted sexual dysfunction to determine the optimal vaginal tone and volume for satisfactory female sexual function. Because of the correlations between the FSFI pain score and vaginal resting tone and vaginal volume, we aimed to determine which variables classified subjects with and without pain-related sexual dysfunction based on an FSFI pain score of 2.5 points. We conducted a decision tree analysis, and the cutoff value was determined. The cutoff point of vaginal resting tone for pain-related sexual dysfunction was >15.2 mmHg; vaginal volume was not involved in the decision tree model. Previous studies reported that the PFMs could become overactive in dyspareunia [34,35]. Thus, modifying the PFM tone should be considered for treating dyspareunia. Our findings might help establish quantitative goals and guidelines for therapy.
The present study has several limitations. First, the data about other psychosocial factors, such as depression, anxiety, or other psychological disorders that could cause female sexual dysfunction were not measured. Second, in addition to psychosocial factors, numerous factors including age, obesity, menopausal status, educational level, income level, interactions with a partner, and overall physical health could affect female sexual function [23,24]. Third, because the study subjects were limited to patients with SUI, it is difficult to generalize the results to the overall female population. Fourth, our small sample size is another notable limitation. Further study is needed to verify the generalizability of our results. Moreover, because decision tree techniques help detect interesting patterns and relationships hidden in a large volume of data, further studies with larger sample sizes are needed. Since the possible causes of female sexual dysfunction are multifactorial, it is vital to explore the relationship between more variables and female sexual function.
The results of the present study determined that PFM strength and vaginal morphology are associated with female sexual function in women with SUI. The desire, arousal, satisfaction, and total FSFI scores and PFM strength were sufficiently correlated. The FSFI pain score and vaginal resting tone and volume were also correlated. Thus, PFM strengthening or training should be considered the first strategy to improve female sexual function. Further, because of the relationship between vaginal morphology and the FSFI pain score, surgical decisions [36] regarding vaginal rejuvenation need to be carefully considered.
Notes
Conflict of interest
The authors declare that they have no potential conflicts of interest with respect to the research, authorship, and publication of this article. The results are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval
The study was approved by the Institutional Review Board at Yonsei University, Mirae Campus (Wonju, Korea; No. 1041849-201904-BM-050-01).
ACKNOWLEDGMENTS
We would like to thank all of the participants for their time and commitment to the present study.
References
1. Moore RD, Miklos JR. Vaginal reconstruction and rejuvenation surgery: is there data to support improved sexual function? AJCS. 2012; 29:97–113.

2. Griffiths A, Watermeyer S, Sidhu K, Amso NN, Nix B. Female genital tract morbidity and sexual function following vaginal delivery or lower segment caesarean section. J Obstet Gynaecol. 2006; 26:645–9.

3. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: a prospective study. J Sex Me. 2008; 5:1915–22.

4. Safarinejad MR, Kolahi AA, Hosseini L. RETRACTED: the effect of the mode of delivery on the quality of life, sexual function, and sexual satisfaction in primiparous women and their husbands. J Sex Med. 2009; 6:165–67.

5. Hwang UJ, Kwon OY, Lee MS. Effects of surface electrical stimulation during sitting on pelvic floor muscle function and sexual function in women with stress urinary incontinence. Obstet Gynecol Sci. 2020; 63:370–8.

6. Abramov Y, Gandhi S, Botros SM, Goldberg RP, Sherman W, Rurak M, et al. Do alterations in vaginal dimensions after reconstructive pelvic surgeries affect the risk for dyspareunia? Am J Obstet Gynecol. 2005; 192:1573–7.

7. Weber AM, Walters MD, Piedmonte MR. Sexual function and vaginal anatomy in women before and after surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2000; 182:1610–5.

8. Zahariou AG, Karamouti MV, Papaioannou PD. Pelvic floor muscle training improves sexual function of women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:401–6.

9. Bø K, Talseth T, Vinsnes A. Randomized controlled trial on the effect of pelvic floor muscle training on quality of life and sexual problems in genuine stress incontinent women. Acta Obstet Gynecol Scand. 2000; 79:598–603.

10. Messelink B, Benson T, Berghmans B, Bø K, Corcos J, Fowler C, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005; 24:374–80.

11. Haylen BT, De Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010; 29:4–20.

12. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009; 41:1149–60.

13. Hwang UJ, Lee MS, Jung SH, Ahn SH, Kwon OY. Relationship between sexual function and pelvic floor and hip muscle strength in women with stress urinary incontinence. Sex Med. 2021; 9:100325.

14. Hwang UJ. Is there an association between the quality of life of incontinence symptoms and the hip joint muscles strength in women with stress urinary incontinence? J Musculoskelet Sci Technol. 2022; 6:15–21.

15. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The female sexual function index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000; 26:191–208.

16. Kim HY, So HS, Park KS, Jeong SJ, Lee JY, Ryu SB. Development of the Korean-version of female sexual function index (FSFI). Korean J Androl. 2002; 50–6.
17. Bø K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005; 85:269–82.

18. Devreese A, Staes F, De Weerdt W, Feys H, Van Assche A, Penninckx F, et al. Clinical evaluation of pelvic floor muscle function in continent and incontinent women. Neurourol Urodyn. 2004; 23:190–7.

19. Albrich S, Steetskamp J, Knoechel SL, Porta S, Hoffmann G, Skala C. Assessment of pelvic floor muscle contractility: digital palpation versus 2D and 3D perineal ultrasound. Arch Gynecol Obstet. 2016; 293:839–43.

20. Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed. Upper Saddle River: Pearson/Prentice Hall;2009.
21. Hwang UJ, Kwon OY, Yi CH, Jeon HS, Weon JH, Ha SM. Predictors of upper trapezius pain with myofascial trigger points in food service workers: the STROBE study. Medicine (Baltimore). 2017; 96:e7252.
22. Jiann BP, Su CC, Yu CC, Wu TT, Huang JK. Risk factors for individual domains of female sexual function. J Sex Med. 2009; 6:3364–75.

23. Addis IB, Van Den Eeden SK, Wassel-Fyr CL, Vittinghoff E, Brown JS, Thom DH, et al. Sexual activity and function in middle-aged and older women. Obstet Gynecol. 2006; 107:755–64.

24. Chedraui P, Perez-Lopez FR, San Miguel G, Avila C. Assessment of sexuality among middle-aged women using the female sexual function index. Climacteric. 2009; 12:213–21.

25. Barbara G, Facchin F, Buggio L, Alberico D, Frattaruolo MP, Kustermann A. Vaginal rejuvenation: current perspectives. Int J Womens Health. 2017; 9:513–9.

26. Ferreira CH, Dwyer PL, Davidson M, De Souza A, Ugarte JA, Frawley HC. Does pelvic floor muscle training improve female sexual function? A systematic review. Int Urogynecol J. 2015; 26:1735–50.

27. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J Pelvic Floor Dysfunct. 2000; 11:361–76.

28. Giuseppe PG, Pace G, Vicentini C. Sexual function in women with urinary incontinence treated by pelvic floor transvaginal electrical stimulation. J Sex Med. 2007; 4:702–7.

29. Mercier J, Tang A, Morin M, Khalifé S, Lemieux MC, Reichetzer B, et al. Test-retest reliability of clitoral blood flow measurements using color Doppler ultrasonography at rest and after a pelvic floor contraction task in healthy adult women. Neurourol Urodyn. 2018; 37:2249–56.

30. Ma Y, Qin H. Pelvic floor muscle exercises may improve female sexual function. Med Hypotheses. 2009; 72:223.

31. Swift S, Woodman P, O’Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic organ support study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005; 192:795–806.

32. Schimpf MO, Harvie HS, Omotosho TB, Epstein LB, Jean-Michel M, Olivera CK, et al. Does vaginal size impact sexual activity and function? Int Urogynecol J. 2010; 21:447–52.

33. Kim-Fine S, Antosh DD, Balk EM, Meriwether KV, Kanter G, Dieter AA, et al. Relationship of postoperative vaginal anatomy and sexual function: a systematic review with meta-analysis. Int Urogynecol J. 2021; 32:2125–34.

34. Goldfinger C, Pukall CF, Gentilcore-Saulnier E, McLean L, Chamberlain S. A prospective study of pelvic floor physical therapy: pain and psychosexual outcomes in provoked vestibulodynia. J Sex Med. 2009; 6:1955–68.
35. Piassarolli VP, Hardy E, Andrade NF, Ferreira Nde O, Osis MJ. Pelvic floor muscle training in female sexual dysfunctions. Rev Bras Ginecol Obstet. 2010; 32:234–40.
Fig. 1.
Correlation between pain domain scores in the female sexual function index and vaginal resting tone (Shapes: O=pain score greater than 2.5 points, X=pain score less than 2.5 points; colors: pain domain scores in FSFI). FSFI, female sexual function index; PFM, pelvic floor muscle.
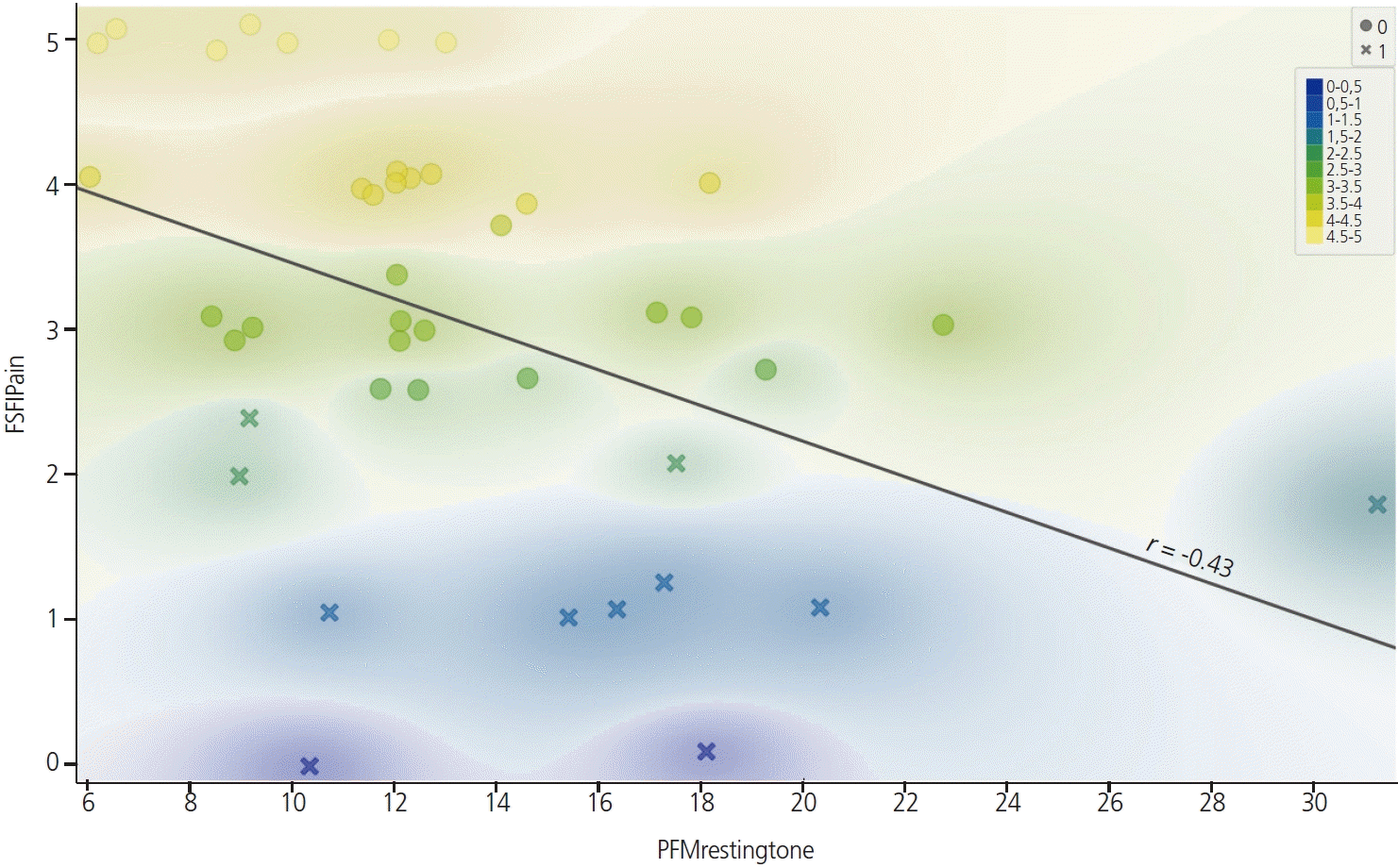
Fig. 2.
Classification tree for the presence of pain-related sexual dysfunction based on a pain score of 2.5 points in the female sexual function index.
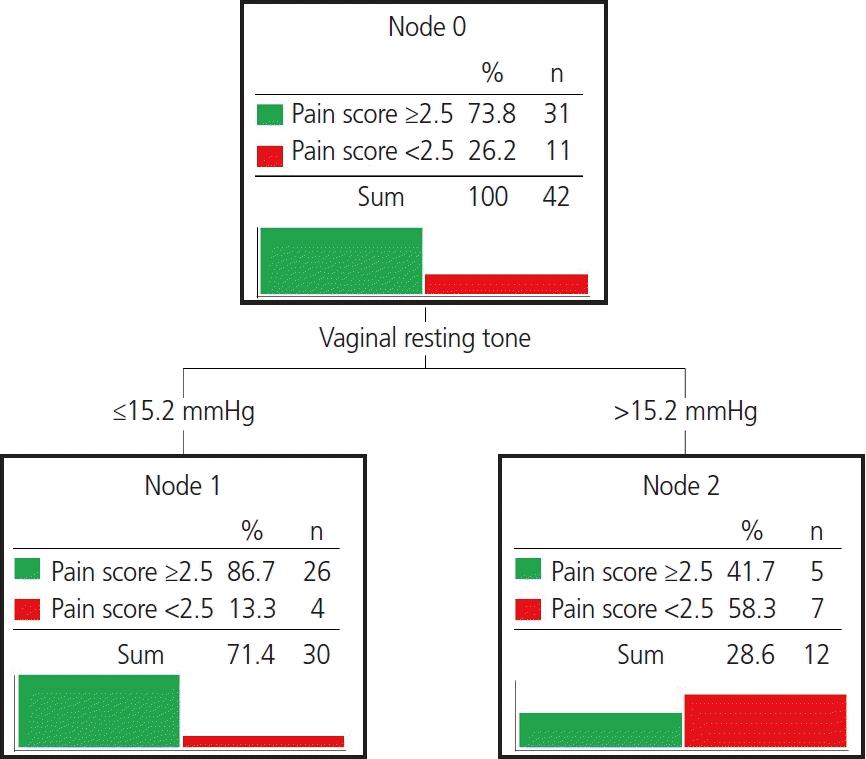
Table 1.
Characteristics of the participants
Table 2.
Descriptive statistics of pelvic floor muscle strength, vaginal resting tone and volume, and the female sexual function index score
Table 3.
The correlation coefficients between pelvic floor muscle strength, vaginal resting tone, vaginal volume, and female sexual function score
|
PFM strength |
Vaginal resting tone |
Vaginal volume |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| FSFIb-desire | 0.397 | 0.009a) | -0.090 | 0.572 | -0.024 | 0.879 |
| FSFI-arousal | 0.388 | 0.011a) | -0.035 | 0.825 | 0.085 | 0.593 |
| FSFI-orgasm | 0.155 | 0.328 | 0.074 | 0.642 | 0.095 | 0.548 |
| FSFI-lubrication | -0.154 | 0.331 | -0.041 | 0.795 | -0.164 | 0.299 |
| FSFI-satisfaction | 0.326 | 0.035a) | 0.100 | 0.529 | 0.122 | 0.440 |
| FSFI-pain | -0.134 | 0.397 | -0.432 | 0.004a) | 0.332 | 0.032a) |
| FSFI-total score | 0.315 | 0.042a) | -0.165 | 0.297 | 0.185 | 0.242 |
Table 4.
Results of stepwise multiple regression analyses determining the coefficients of independent model variables
| R2 | F | P | Model | B | β | |
|---|---|---|---|---|---|---|
| FSFI-desire | 0.201 | 4.907 | 0.013a) | Age | -0.036 | -0.317 |
| PFM strength | 0.298 | 0.311 | ||||
| Constant | 3.181 | |||||
| FSFI-arousal | 0.119 | 5.379 | 0.026a) | PFM strength | 0.421 | 0.344 |
| Constant | 1.431 | |||||
| FSFI-orgasm | No variables entered the model | |||||
| FSFI-lubrication | No variables entered the model | |||||
| FSFI-satisfaction | 0.108 | 4.864 | 0.033a) | PFM strength | 0.406 | 0.329 |
| Constant | 1.691 | |||||
| FSFI-pain | 0.187 | 9.195 | 0.004a) | Vaginal resting tone | -0.124 | -0.432 |
| Constant | 4.704 | |||||
| FSFI-total score | 0.123 | 5.623 | 0.023a) | Age | -0.155 | -0.351 |
| Constant | 20.996 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download