Abstract
Objective
To compare the diagnostic accuracy of a handheld colposcope (Gynocular) versus a standard colposcope in women with abnormal cervical cytology or visual Inspection with acetic acid positivity.
Methods
This crossover randomized clinical trial was conducted in Pondicherry, India, and included 230 women who were referred for colposcopy. Swede scores were calculated using both colposcopes, and a cervical biopsy was performed from the most visually abnormal areas. Swede scores were compared with the histopathological diagnosis, which was used as the reference standard. The level of agreement between the two colposcopes was calculated using Kappa (κ) statistics.
Results
The level of agreement of Swede scores between the standard and Gynocular colposcopes was 62.56%, and the κ statistic was 0.43 (P<0.001). Cervical intraepithelial neoplasia (CIN) 2+ (CIN 2, CIN 3, CIN 3+) was diagnosed in 40 (17.4%) women. There were no significant differences between the two colposcopes in terms of sensitivity, specificity, or predictive value for detecting CIN 2+ lesions.
Cervical cancer is a major public health problem in low- and middle-income countries (LMICs). Of the estimated 342,000 deaths from cervical cancer every year, around 90% occur in LMICs [1,2]. The World Health Organization adopted a global strategy to eliminate cervical cancer by 2030 [3]. Effective cervical cancer screening is important to achieve this goal. Colposcopic evaluation is an important step in the diagnosis and management of pre-invasive lesions in women with abnormal cervical cancer screening results. Portable colposcopes, such as Gynocular colposcopes, were developed to provide healthcare personnel with a low-cost, handheld, battery-driven colposcope that enables colposcopy in any setting, including mobile camps and outreach screening programs [4–6]. Very few studies have compared the diagnostic accuracy of a Gynocular colposcope with that of a standard colposcope [4,7,8]. A recent meta-analysis of portable colposcopes emphasized the need for more studies [9]. In this study, we aimed to compare the diagnostic accuracy of the Gynocular and standard colposcopes in detecting cervical intraepithelial neoplasia (CIN) 2+ (CIN 2, CIN 3, CIN 3+) lesions.
This crossover randomized controlled trial was conducted at the Department of Obstetrics and Gynecology, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry, India. The study enrollment period was from January 2020 to March 2021. This study was approved by the Institutional Ethics Committee and registered under the Clinical Trials Registry-India (CTRI/2019/12/022584). In India, visual inspection with acetic acid (VIA) is the most common method of cervical cancer screening, especially in primary health centers, whereas medical college hospitals generally use Pap smears for screening. The VIA is a simple test used in many low-resource countries. Health workers in primary healthcare centers are trained to perform this test, and the results are immediately available. Freshly prepared 4% acetic acid was applied to the cervix by health workers after the insertion of a sterile Cusco self-retaining vaginal speculum. The results were recorded after 1 minute of illumination. The test result was labeled as VIA positive if there was a well-defined, dense acetowhite area with a regular margin in the transformation zone.
Women referred to our colposcopy clinic with abnormal cervical cytology findings or positive VIA reports were included in this study. The exclusion criteria included pregnancy and ongoing vaginal bleeding. Women who agreed to participate in the study and provided informed consent were randomized into two groups: Gynocular and standard colposcopy. Block randomization with varying block sizes generated using a computer was used to randomize the patients in the study arms at a ratio of 1:1. Sequentially numbered, opaque, sealed envelope allocations were used for concealment. All colposcopies were performed by a single specialist consultant trained in colposcopy. Women allocated to the Gynocular and standard colposcopy groups first underwent colposcopy with the Gynocular (Gynius AB, Stockholm, Sweden) and standard colposcopes (Leisegang Optik, Berlin, Germany), respectively. The Swede score was calculated and the most visually abnormal area was recorded [4]. The Swede score considers five characteristics, namely the density of acetic acid uptake, type of margin, size of the lesion, uptake of iodine, and characteristics of blood vessels. Scores of 0, 1, and 2 were assigned to each characteristic. Swede scores of ≥5 indicated probable CIN 2+ (CIN 2, CIN 3, CIN 3+) lesions [4].
A cervical biopsy was not performed during the first colposcopic examination. After a 2-week interval, the patients returned for a follow-up at the colposcopy clinic. During the second visit, the alternative method of colposcopy was performed. The details of earlier colposcopic findings and Swede scores were not revealed to the investigator performing the colposcopy. During the second colposcopy, the Swede score was calculated again, and the most visually abnormal area was noted. Two or more cervical biopsies were obtained from visually abnormal areas for histopathological examination in all women. A single random biopsy was obtained from transformation zone if the colposcopy results were normal.
Colposcopic diagnosis, according to the Swede score (Swede score of ≥5), was compared to histopathological diagnosis, which is considered the gold standard in diagnosing CIN lesions. The histopathological diagnosis of CIN 2+ (CIN 2, CIN 3, CIN 3+) was used as the reference standard in this study. The degree of agreement between the two colposcopic techniques was compared with gold standard biopsy results.
For the sample size calculation, the sensitivity for identifying CIN 2+ was assumed to be 91% using the standard colposcope and 86% using the Gynocular colposcope. The non-inferiority margin for the difference between the two colposcopes was defined as 5%. For an alpha value of 5% and power of 80%, the total sample size was calculated to be 230 (Sealed Envelope Ltd., London, UK). Assuming 10% dropout rate, total sample size was recalculated as 250.
Categorical variables are expressed as frequencies and percentages. Continuous variables are expressed as means and standard deviations or medians with ranges based on the distribution of data. All the statistical analyses were carried out at a 5% level of significance, and P-values <0.05 were considered statistically significant. The diagnostic accuracy was assessed using sensitivity, specificity, and predictive values with corresponding 95% confidence interval (CI). Kappa (κ) statistics were used to compare the level of agreement between Gynocular and standard colposcopy. The software used for analysis was SPSS version 22.0 (IBM Corp, Armonk, NY, USA).
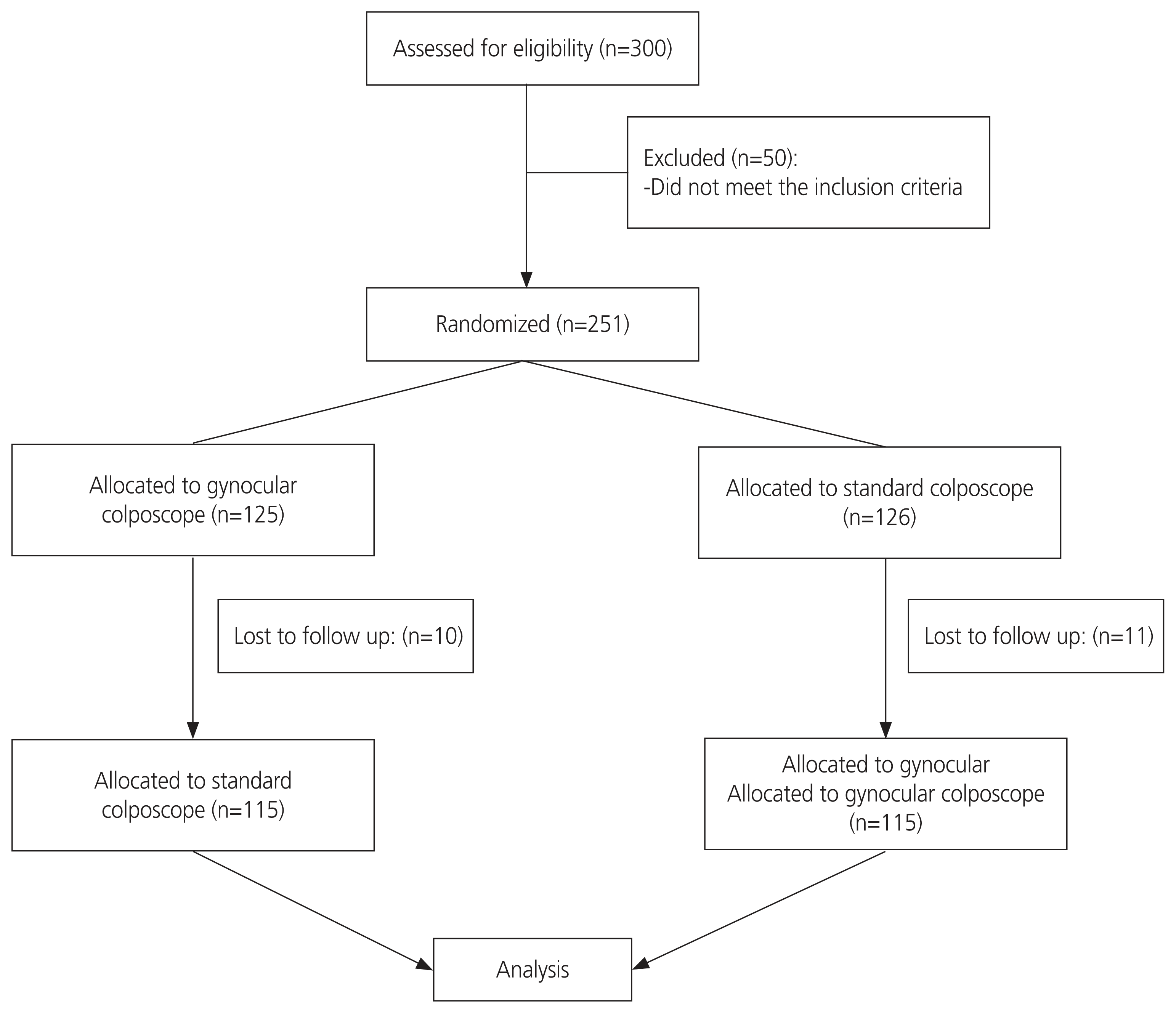
Three hundred women were assessed for eligibility and 251 were included in the study (Fig. 1). The baseline patient characteristics are shown in Table 1. The mean age standard deviation was 43.51±10.8 years. Most of the study population was referred to the colposcopy clinic because of VIA positivity, n=163 (70.8%). Sixty-seven women (29.1%) were referred due to abnormal Pap smear results.
All women underwent a cervical biopsy, irrespective of the Swede score, during the second colposcopy. Cervical biopsy results were normal in 181 women (78.7%) and we identified CIN 1 lesions in nine women (3.9%) and CIN 2+ lesions in 40 women (17.4%). Of these 40 women with CIN 2+, 10 (4.3%) had invasive cervical cancer.
The level of agreement of Swede scores between the standard and Gynocular colposcopes was 62.56%, and the κ statistic was 0.43 (P<0.001), indicating a good level of agreement. There was no significant difference between the Gynocular and standard colposcopes in identifying women with Swede scores of ≥5. The area under the curve (AUC) for detecting CIN 2+ lesions was similar for both colposcopes, as shown in the AUC-receiver operating characteristic curve (Fig. 2).
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of Gynocular colposcopy to detect CIN 2+ were 75% (95% CI, 58.8% to 87.3%), 96.8% (95% CI, 93.3% to 98.8%), 83.3% (95% CI, 67.2% to 93.6%), and 94.8% (95% CI, 90.7% to 97.5%), respectively, for Swede scores of ≥5. Standard colposcopy had a sensitivity, specificity, PPV, and NPV of 80.0% (95% CI, 64.4% to 90.9%), 95.3% (95% CI, 91.2% to 97.8%), 78.0% (95% CI, 62.4% to 89.4%), and 95.8% (95% CI, 91.8% to 98.2%), respectively, to detect CIN 2+ for Swede scores of ≥5 (Tables 2, 3).
The difference in sensitivity observed between the two techniques was found to be 5% (95% CI, 2.2% to 7.7%), suggesting that Gynocular colposcopy was non-inferior to the standard colposcope.
This crossover randomized controlled study compared the diagnostic accuracy of handheld Gynocular and standard colposcopy in detecting CIN 2+ lesions. There were no significant differences between the two colposcopes in terms of their sensitivity, specificity, or predictive value. The correlation of the Swede scores between the two colposcopes was also similar, with a κ coefficient of 0.43, indicating a good level of agreement.
The diagnostic performance of both colposcopes was very good, with both colposcopes having a specificity of >95% at Swede scores of ≥5. The Gynocular colposcope, which is portable, has the added advantage of being low cost and battery-driven, thus enabling colposcopy in outreach screening programs.
Several handheld, rechargeable colposcopes, such as Gynocular colposcopes, have been developed in recent years [4,10–15]. These portable colposcopes may be especially useful in LMICs, where access to stationary colposcopes is limited. The Gynocular colposcope is a hand-held monocular colposcope and has been compared with the standard colposcope in few studies [4,7,8,13]. Kallner et al. [8] conducted a crossover randomized controlled study to evaluate the diagnostic accuracy of Gynocular and standard colposcopy in detecting CIN in 126 women. Both the colposcopes showed a high agreement in Swede scores. There were no significant differences in the sensitivity and specificity for different Swede scores in Gynocular or standard colposcopy in detecting CIN 2+ lesions. Another crossover randomized clinical trial compared these two colposcopes in 540 VIA-positive women [7]. This study also showed a significant level of agreement of Swede scores between the two colposcopes. A crossover, randomized study evaluated the performance of trained nurses in detecting cervical lesions using a stationary colposcope or Gynocular colposcope as compared to doctors [13]. The Swede scores obtained by nurses and doctors using the Gynocular and stationary colposcopes showed high agreement, and there was no difference in detecting cervical lesions in biopsies.
Earlier studies that compared Gynocular and standard colposcopes had several limitations. In these studies, cervical biopsies were not performed in all women, resulting in partial verification bias [9]. In our study, this partial verification bias was eliminated by performing cervical biopsies on all women. Informed consent was obtained from all participants, and the study was approved by the institutional ethics committee. Moreover, in our study, at least 2 cervical biopsies were performed in all women to minimize misclassification [4,9]. Another merit of our study was that the investigator was blinded to earlier colposcopic findings. This was possible because there was 14 days gap between the two colposcopic examinations. All colposcopic examinations were performed by a single experienced investigator, thus eliminating inter-observer bias.
The main limitation of our study was its relatively small sample size. Another limitation was that almost 8% of women were lost to follow up after the first colposcopy and had to be excluded from the study.
The diagnostic accuracy of Gynocular colposcopes is similar to that of standard colposcopes in the detection of CIN 2+ lesions. Gynocular colposcopes have a good level of agreement with standard colposcopes when the Swede score is used and can be used as an alternative to standard colposcopes in low-resource settings.
Notes
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.

2. Ghalavandi S, Heidarnia A, Zarei F, Beiranvand R. Knowledge, attitude, practice, and self-efficacy of women regarding cervical cancer screening. Obstet Gynecol Sci. 2021; 64:216–25.

3. World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem [Internet]. Geneva: World Health Organization;c2020. [cited 2020 Nov 17]. Available from: https://www.who.int/publications/i/item/9789240014107.
4. Ngonzi J, Bajunirwe F, Wistrand C, Mayanja R, Altman D, Thorsell M, et al. Agreement of colposcope and gynocular in assessment of cervical lesions by swede score: a randomized, crossover pilot trial. J Low Genit Tract Dis. 2013; 17:372–7.
5. Basu P, Banerjee D, Mittal S, Mandal R, Ghosh I, Das P, et al. Evaluation of a compact, rechargeable, magnifying device to triage VIA and HPV positive women in a cervical cancer screening program in rural India. Cancer Causes Control. 2016; 27:1253–9.

6. Newman H, Hu J, Li X, He J, Bradford L, Shan S, et al. Evaluation of portable colposcopy and human papillomavirus testing for screening of cervical cancer in rural China. Int J Gynecol Cancer. 2019; 29:23–7.

7. Nessa A, Wistrand C, Begum SA, Thuresson M, Shemer I, Thorsell M, et al. Evaluation of stationary colposcope and the gynocular, by the Swede score systematic colposcopic system in VIA positive women: a crossover randomized trial. Int J Gynecol Cancer. 2014; 24:339–45.

8. Kallner HK, Persson M, Thuresson M, Altman D, Shemer I, Thorsell M, et al. Diagnostic colposcopic accuracy by the gynocular and a stationary colposcope. Int J Technol Assess Health Care. 2015; 31:181–7.

9. Taghavi K, Rohner E, Basu P, Low N, Rutjes A, Bohlius J. Screening test accuracy of portable devices that can be used to perform colposcopy for detecting CIN2+ in low- and middle-income countries: a systematic review and meta-analysis. BMC Womens Health. 2020; 20:253.

10. Lam CT, Mueller J, Asma B, Asiedu M, Krieger MS, Chitalia R, et al. An integrated strategy for improving contrast, durability, and portability of a pocket colposcope for cervical cancer screening and diagnosis. PLoS One. 2018; 13:e0192530.

11. Sato M, Shintani D, Hanaoka M, Sato S, Miwa M, Ogasawara A, et al. A pilot study of mobile digital colposcopy in Japanese patients with cervical intraepithelial neoplasm. Mol Clin Oncol. 2021; 15:207.

12. Singh V, Parashari A, Gupta S, Sodhani P, Sehgal A. Performance of a low cost magnifying device, magnivisualizer, versus colposcope for detection of pre-cancer and cancerous lesions of uterine cervix. J Gynecol Oncol. 2014; 25:282–6.

13. Nessa A, Roy JS, Chowdhury MA, Khanam Q, Afroz R, Wistrand C, et al. Evaluation of the accuracy in detecting cervical lesions by nurses versus doctors using a stationary colposcope and gynocular in a low-resource setting. BMJ Open. 2014; 4:e005313.

Fig. 2
Receiver operating characteristic curves comparing both colposcopes in identifying women with CIN 2+ lesions. CIN, cervical intraepithelial neoplasia.
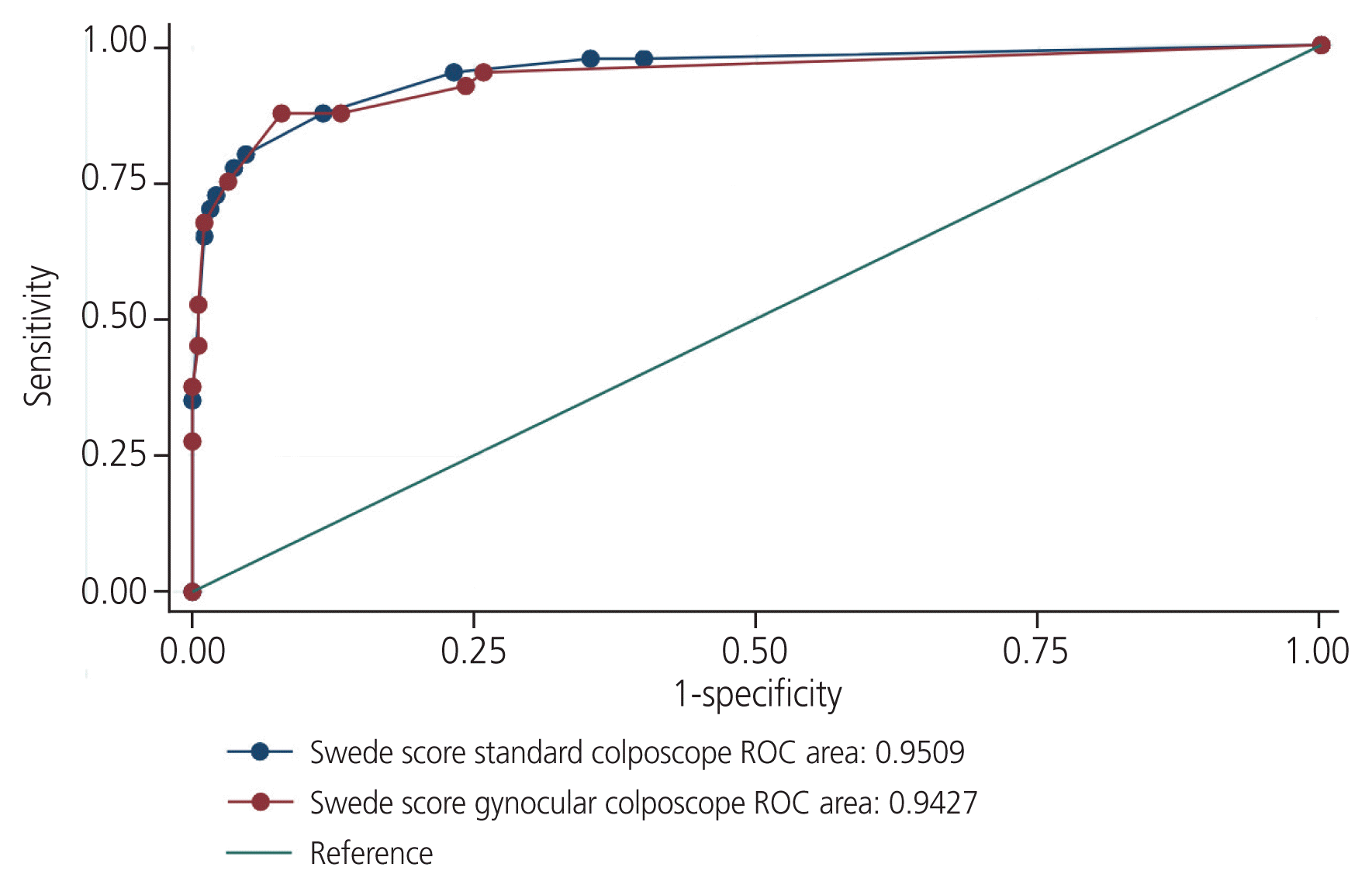
Table 1
Baseline characteristics of study population
Table 2
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of gynocular colposcope to detect CIN 2+ lesions for different cut-off levels of Swede score
Table 3
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of standard colposcope to detect CIN 2+ lesions for different cut-off levels of Swede score




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download