Abstract
Cognitive dysfunction is relatively less considered a complication of hypertension. However, there is sufficient evidence to show that high blood pressure in middle age increases the risk of cognitive decline and dementia in old age. The greatest impact on cognitive function in those with hypertension is on executive or frontal lobe function, similar to the area most damaged in vascular dementia. Possible cognitive disorders associated with hypertension are vascular dementia, Alzheimer disease, and Lewy body dementia, listed in decreasing strength of association. The pathophysiology of cognitive dysfunction in individuals with hypertension includes brain atrophy, microinfarcts, microbleeds, neuronal loss, white matter lesions, network disruption, neurovascular unit damage, reduced cerebral blood flow, blood-brain barrier damage, enlarged perivascular damage, and proteinopathy. Antihypertensive drugs may reduce the risk of cognitive decline and dementia. Given the high prevalence of dementia and its impact on quality of life, treatment of hypertension to reduce cognitive decline may be a clinically relevant intervention.
Go to : 
Hypertension is a serious medical condition that significantly increases the risk of cardiovascular, cerebral, renal, and other organ dysfunction [1]. Cognitive impairment is comparatively less considered to be an adverse effect of hypertension. However, accumulating evidence supports the causal role of hypertension in cognitive decline beyond its relationship with stroke [2]. Cognitive decline in old age is regarded as an irreversible condition due to degenerative changes, and in many cases, it reduces quality of life and is difficult to treat in diagnosed patients [3]. However, this cognitive decline is affected by many other factors in addition to normal age-related degenerative changes, and hypertension is one of the most important risk factors because it can be controlled and modified and has a high prevalence [4]. Therefore, useful research evidence should be obtained to review and summarize the cognitive dysfunction in patients with hypertension. The purpose of this study is to examine the characteristics of cognitive dysfunction in patients with hypertension and to summarize studies showing the association of hypertension with cognitive disorders and how hypertension causes pathophysiological changes in the brain that lead to cognitive dysfunction.
Go to : 
Epidemiological data from the Framingham study [5] suggest that there is no association between blood pressure (BP) and co-measured cognitive ability. However, a longitudinal reanalysis of the data showed that the 20-year mean BP was inversely related to cognitive ability [5]. A midlife hypertension and 20-year cognitive cohort study demonstrated that only high systolic BP in midlife, not increased systolic BP in late life was associated with more cognitive decline during the 20-year study [6]. Other cohort studies have also shown that midlife hypertension is a significant predictor of both cognitive dysfunction and morphological changes in the brain [7,8]. A retrospective cohort study including 721 individuals also found that midlife hypertension was associated with late-life dementia [9,10]. The Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) study, including 1,449 participants aged 65 to 79 years with an average follow-up of over 21 years, suggested that midlife hypertension was associated with an increased risk of dementia in late life [11]. Another study indicated that hypertension in middle age increases the risk of mild cognitive impairment (MCI). A population-based study, using the same cohort of CAIDE study, suggested that midlife hypertension is associated with the development of MCI in late life [12]. The Atherosclerosis Risk in Communities (ARIC) cohort study showed that hypertension and prehypertension in midlife are associated with an increased risk of dementia in late life [9]. The association between midlife hypertension and dementia was also suggested by the Honolulu-Asia Aging Study of 3,703 participants, which showed a consistent association between Alzheimer disease (AD) and vascular dementia [13]. A recent meta-analysis that included 209 prospective studies also showed that midlife hypertension has a stronger association than late-life hypertension. In that meta-analysis, midlife hypertension was associated with an excess risk of 1.19 to 1.55 times that of cognitive impairment. The dose-response relationship was also analyzed, and a midlife systolic BP greater than 130 mmHg was associated with an increased risk of cognitive disorders [14]. The follow-up Honolulu-Asia Aging Study, including 2,505 male participants aged 71 to 93 years, showed that pulsatile pressure was not associated with the incidence of dementia; however, midlife systolic BP was the strongest predictor of dementia incidence [15]. Another study investigated the interactive effects of apolipoprotein E ε4 (APOE-ε4) and hypertension on cognitive decline and brain atrophy. In that study, hypertension was associated with early cognitive decline and brain atrophy in mid-to-late life, particularly in APOE-ε4 carriers [16].
In addition, an U-shaped relationship between hypertension and dementia incidence was reported in a longitudinal population-based study [17]. Low diastolic BP in late life is also associated with an increased risk of AD [18].
Go to : 
Cognitive impairments in hypertension can occur across multiple neuropsychological domains, including learning and memory, attention, abstract reasoning, mental flexibility, psychomotor skills, and visuospatial functioning [19]. Although cognitive impairment associated with hypertension may be global, when a more detailed neuropsychological battery is implemented and hypertension-specific effects are considered and compared, the greatest impact of hypertension is on executive function [6], motor speed, and attention [20]. Other studies have reported that hypertensive cerebrovascular disease generally causes damage to the prefrontal subcortex, making it difficult to form goals, abstract, initiate, plan, organize, and sequence [19,21,22]. These characteristic cognitive domains are thought to be involved in subcortical diseases such as classical vascular disease and pure vascular dementia [23]. Although these impairments in executive or prefrontal lobe function usually mask the memory impairment characteristic of AD, impairments in these two cognitive functions often co-occur. Memory impairments in hypertension often tend to be characterized by impairments in recall, but relatively intact recognition, benefit from cues and mild forgetfulness [13].
In a cross-sectional cohort study of 67 patients aged 60 years and older who were hypertensive with MCI or subjective cognitive problems, markers of beta-amyloid retention were associated with worsening episodic memory. In contrast, high white matter intensity, a marker of subcortical ischemic injury, was not associated with performance in any cognitive domain [24]. Because the function of the prefrontal cortex is dependent on the integrity of the cortical-striatal loop through the prefrontal white matter, this type of lesion is very likely to cause a decline in working memory, executive function, and other cognitive abilities assisted by the prefrontal cortex [25]. Therefore, it can be hypothesized that subcortical cerebrovascular disease is more likely to cause cognitive symptoms that are distinguishable from those of AD [26]. Neuropsychological studies of clinically diagnosed patients have reported that compared to patients with AD, patients with vascular dementia performed better on memory tests and poorer on executive function tests. This observation suggests that predominant executive dysfunction may serve as a useful diagnostic marker of vascular dementia. However, a study of 62 autopsy cases showed that major memory impairment was present in 71% of AD cases and predominant executive dysfunction accounted for only 45% of cerebrovascular diseases [26]. In contrast, within large groups that are likely to overlap with the pathology of AD, distinguishing subtypes of dementia based on patterns in impaired cognitive domains is difficult in individuals with mixed MCI and dementia conditions. Finally, a recent meta-analysis showed an association between midlife hypertension and overall cognitive and executive function but not memory [14].
Go to : 
Hypertension has been implicated in various neurocognitive disorders ranging from mild to major [27]. A clear pathophysiological process of vascular dementia in hypertension has been reported [28]. However, hypertension has also been considered a risk factor for AD, although this relationship has not been as clearly elucidated as that between hypertension and vascular dementia [29,30]. In one study, 1,385 participants with a diagnosis of MCI were analyzed to determine whether the degree of high BP was associated with a faster decline in certain cognitive function domains. There were significant main effects of high BP (systolic BP of ≥140 mmHg or diastolic BP of ≥90 mmHg) on neuropsychological measures of visuomotor sequencing, set shifting, and naming. It showed that high BP is associated with a faster decline in cognitive function in people at risk of dementia [31]. Another prospective community-based cohort study also showed that hypertension was related to an increased risk of MCI, and this relationship was stronger in patients with non-amnestic MCI [32].
A significant association was found between frontotemporal dementia and type 2 diabetes [33]. However, in a cohort study, hypertension and other cerebrovascular risk factors were not identified as risk factors for frontotemporal dementia [34]. In a population-based study, hypertension significantly increased the risk of both AD and diffuse dementia with Lewy body (DLB). However, the relationship between hypertension and DLB has been less studied [35]. Finally, a retrospective study investigating BP difference among individuals with different dementia disorders, found that for those patients over 80 years of age, BP did not differ as a function of various dementia disorders [27,34].
In summary, there is strong evidence of an association between vascular dementia and hypertension. However, high BP may contribute to other neurocognitive disorders, such as AD and DLB. Hypertension can negatively affect MCI and shorten the transition to major neurocognitive mpairment (Table 1).
Cognitive domains and disorders associated with hypertension
Go to : 
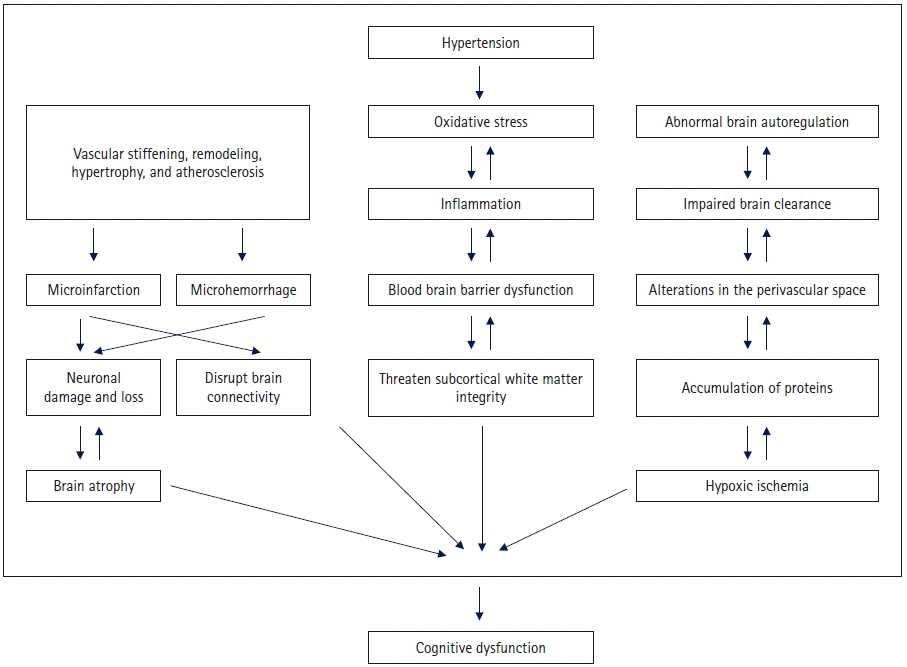
Chronic hypertension is associated with adaptive and degenerative structural changes in the cerebral vessels. These structural changes initially reflect an adaptive response to protect the downstream microvessels from increased transwall pressure. However, this process becomes maladaptive over time. The mechanism of pathological changes associated with hypertension includes vascular remodeling and stiffening, cerebral autoregulation impairment, microbleeding and microinfarction, white matter lesions, lacunar infarction, amyloid angiopathy, and cerebral atrophy [23,36-43]. Brain atrophy, microinfarction, and microhemorrhage can cause nerve cell loss and impaired brain function. In addition, microinfarction and microhemorrhage disrupt brain connectivity and reduce efficiency of the network. Damage to white matter specifically reduces network connectivity, particularly in the thalamic cortical circuit [23,44]. Alterations in the perivascular space can impair brain clearance and promote the accumulation of proteins in the brain and blood vessels. Dysfunction of the neurovascular unit is linked to vascular dysfunction and blood-brain barrier damage [23,45]. Oxidative stress, hypoxic ischemia, inflammation, and blood-brain barrier dysfunction are important vascular factors that threaten the integrity and function of the subcortical white matter. There are possible regional differences in the effects of hypertension on white matter, and white matter in the frontal lobe may be more affected [46,47].
Epidemiological studies have shown that hypertension is a risk factor for vascular cognitive impairment and AD. The effects of hypertension on vascular pathologies have been quite clearly established; however, its impact on AD pathologies is unclear and controversial. In a prospective cohort study of 346 subjects, a cumulative number of midlife vascular risk factors were associated with elevated brain amyloid deposition in late life [48]. However, hypertension itself was not significantly associated with elevated amyloid deposition. Another study evaluating the effect of vascular health on AD imaging biomarkers showed that vascular health had a significantly larger effect on neurodegeneration than on amyloid deposition [49]. In a study of 1,300 deceased participants, some associations were found between systolic BP and neurofibrillary tangles [49,50]. A cerebrospinal fluid biomarker study also found that hypertension was not associated with amyloid-beta 1–42, and that APOE did not have a modifying effect. However, hypertension was directly related to tau, and ptau-181 was modified by the APOE genotype [51]. These findings suggest that hypertension is associated with AD but affects the disease through a pathway different from that involved in amyloid pathology. Unlike clinical studies, experimental studies have suggested that hypertension can contribute to both amyloid and tau pathology in AD, although whether hypertension is a contributor or pathological factor needs to be determined [52-55].
In a longitudinal, prospective, population-based study, men with hypertension who were middle-aged and did not receive antihypertensive treatment had a higher risk of hippocampal atrophy than age-matched men with normal BP. Treatment with antihypertensives reduced the risk associated with these high BPs [56]. These findings suggest that the management of hypertension to lower the risk of AD is important, although the magnitude of the effect of hypertension on AD could be smaller than that on vascular dementia (Fig. 1).
Go to : 
Data from randomized controlled clinical trials on the efficacy of antihypertensive therapy for the prevention of dementia are contradictory [57-63]. A meta-analysis, including nine randomized controlled trials with 34,994 participants (>60 years) treated for at least 12 months, showed that antihypertensive treatment could reduce cognitive decline with modest effect sizes and did not worsen cognitive dysfunction [64]. Another meta-analysis demonstrated the benefits of antihypertensives. Compared to controls, a drop in BP was significantly associated with a reduced risk of dementia or cognitive impairment (12 trials, 92,135 participants) [65]. A recent review argued that the correlation between BP and cognitive decline was U-shaped and varied according to age. That is, high BP in middle age may be related to cognitive decline in old age, while hypertension in old age may be less related to cognitive decline. In addition, excessively low BP may be associated with cognitive decline; therefore, these U-shaped associations may neutralize the outcomes [18,66]. Subdivided age-specific studies and studies that removed these confounding factors more consistently showed the effects of hypertension on cognitive dysfunction and the ability of antihypertensive drugs to lessen cognitive decline. There is evidence that all effective BP-lowering drugs, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, and aldosterone antagonists, can be used to prevent cognitive decline in patients with hypertension and there is no evidence for a difference in effectiveness between these antihypertensives [58,60,67-74]. In addition, a meta-analysis of AD risk factors investigated the relationship between antihypertensive drugs and the risk of cognitive decline. This meta-analysis found that antihypertensive treatment was one of the protective factors for AD with grade I evidence (pooled population of >5,000 individuals) [75].
Go to : 
In this review, we discussed previous studies on hypertension-related cognitive dysfunction. Epidemiological data have shown that midlife hypertension is associated with late-life cognitive impairment. These cognitive disorders encompass both mild and major neurocognitive disorders. Hypertension showed the strongest association with vascular dementia, followed by AD and DLB. Frontotemporal dementia showed no evidence of an association. Hypertension was found to be the most consistent risk factor for dementia only at a certain age; that is, middle-aged hypertension was a risk factor for late-life dementia. In addition, hypotension in old age can be a risk factor for cognitive decline along with hypertension; therefore, BP and cognitive decline have an U-shape relationship. Considering these two characteristics will enable a more consistent interpretation of the mixed research results. Cognitive decline related to hypertension is possible in all areas; however, the decline in executive function related to the frontal lobe is particularly noticeable. This is consistent with the characteristics of vascular dementia, but there are many cases in which AD and vascular dementia coexist in clinical practice, making it difficult to diagnose patients with only these cognitive function characteristics. Many studies have been conducted on the mechanisms by which hypertension causes cognitive decline, including brain atrophy, microinfarcts, microbleeds, neuronal loss, white matter lesions, network disruption, neurovascular unit damage, reduced cerebral blood flow, blood-brain barrier damage, enlarged perivascular damage, and proteinopathy. Medications, lifestyle, and comorbidities, such as diabetes and hyperlipidemia, may have indirect effects in addition to the direct pathophysiology of high BP causing cognitive dysfunction. Hypertension is also a risk factor for other diseases that cause cognitive decline, such as chronic kidney disease or heart failure. Although these factors were identified as confounding factors in the studies included in this review and corrections were made, a more detailed study and review are required in the future. Recent meta-analyses have demonstrated that antihypertensive drugs can reduce the risk of cognitive decline and dementia. Therefore, hypertension is an important risk factor for cognitive decline and dementia and is clinically meaningful because it can be controlled and treated. Considering the high prevalence of both conditions and the serious impact of cognitive function on quality of life, active and timely management of hypertension is required. Additional research is needed, such as a study to determine the optimal BP according to age, which would be helpful for cognitive function, and a therapeutic alternative to address the pathophysiological changes in the brain caused by hypertension.
Go to : 
References
1. Lackland DT, Weber MA. Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol. 2015; 31:569–71.
2. Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003; 5:255–61.
3. Fratiglioni L, De Ronchi D, Agüero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999; 15:365–75.
4. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005; 365:217–23.
5. Farmer ME, Kittner SJ, Abbott RD, Wolz MM, Wolf PA, White LR. Longitudinally measured blood pressure, antihypertensive medication use, and cognitive performance: the Framingham Study. J Clin Epidemiol. 1990; 43:475–80.
6. Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014; 71:1218–27.
7. Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998; 51:986–93.
8. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA. 1995; 274:1846–51.
9. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017; 74:1246–54.
10. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005; 64:277–81.
11. Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005; 62:1556–60.
12. Kivipelto M, Helkala EL, Hänninen T, Laakso MP, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001; 56:1683–9.
13. Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000; 21:49–55.
14. Ou YN, Tan CC, Shen XN, Xu W, Hou XH, Dong Q, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020; 76:217–25.
15. Freitag MH, Peila R, Masaki K, Petrovitch H, Ross GW, White LR, et al. Midlife pulse pressure and incidence of dementia: the Honolulu-Asia Aging Study. Stroke. 2006; 37:33–7.
16. Nation DA, Preis SR, Beiser A, Bangen KJ, Delano-Wood L, Lamar M, et al. Pulse pressure is associated with early brain atrophy and cognitive decline: modifying effects of APOE-ε4. Alzheimer Dis Assoc Disord. 2016; 30:210–5.
17. Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001; 322:1447–51.
18. Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009; 8:61–70.
19. Waldstein SR, Jennings JR, Ryan CM, Muldoon MF, Shapiro AP, Polefrone JM, et al. Hypertension and neuropsychological performance in men: interactive effects of age. Health Psychol. 1996; 15:102–9.
20. Goldstein FC, Hajjar IM, Dunn CB, Levey AI, Wharton W. The relationship between cognitive functioning and the JNC-8 guidelines for hypertension in older adults. J Gerontol A Biol Sci Med Sci. 2017; 72:121–6.
21. Erkinjuntti T. Subcortical vascular dementia. Cerebrovasc Dis. 2002; 13(Suppl 2):58–60.
22. Desmond DW. Cognition and white matter lesions. Cerebrovasc Dis. 2002; 13(Suppl 2):53–7.
23. Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019; 124:1025–44.
24. Smith EE, Muzikansky A, McCreary CR, Batool S, Viswanathan A, Dickerson BC, et al. Impaired memory is more closely associated with brain beta-amyloid than leukoaraiosis in hypertensive patients with cognitive symptoms. PLoS One. 2018; 13:e0191345.
25. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990; 13:266–71.
26. Reed BR, Mungas DM, Kramer JH, Ellis W, Vinters HV, Zarow C, et al. Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain. 2007; 130(Pt 3):731–9.
27. Hestad K, Engedal K, Horndalsveen P, Strand BH. Blood pressure in different dementia disorders, mild cognitive impairment, and subjective cognitive decline. Front Aging Neurosci. 2020; 12:257.
28. Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000; 31:1494–501.
29. Phelps EB, Swantek S. Meta-analysis of modifiable risk factors of Alzheimer’s disease. In : Tampi RR, Tampi DJ, Young JJ, Balasubramaniam M, Joshi P, editors. Essential reviews in geriatric psychiatry. New York: Springer Cham;2022. p. 349–53.
30. Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011; 22:646–59.
31. Goldstein FC, Levey AI, Steenland NK. High blood pressure and cognitive decline in mild cognitive impairment. J Am Geriatr Soc. 2013; 61:67–73.
32. Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007; 64:1734–40.
33. Golimstok A, Cámpora N, Rojas JI, Fernandez MC, Elizondo C, Soriano E, et al. Cardiovascular risk factors and frontotemporal dementia: a case-control study. Transl Neurodegener. 2014; 3:13.
34. Atkins ER, Bulsara MK, Panegyres PK. Cerebrovascular risk factors in early-onset dementia. J Neurol Neurosurg Psychiatry. 2012; 83:666–7.
35. Cheng CK, Tsao YC, Su YC, Sung FC, Tai HC, Kung WM. Metabolic risk factors of Alzheimer’s disease, dementia with Lewy bodies, and normal elderly: a population-based study. Behav Neurol. 2018; 2018:8312346.
36. Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017; 120:449–71.
37. Watase H, Sun J, Hippe DS, Balu N, Li F, Zhao X, et al. Carotid artery remodeling is segment specific: an in vivo study by cardiovascular magnetic resonance imaging. Circulation. 2015; 132(Suppl 3):A18848.
38. Iulita MF, Noriega de la Colina A, Girouard H. Arterial stiffness, cognitive impairment and dementia: confounding factor or real risk? J Neurochem. 2018; 144:527–48.
39. Ogola BO, Zimmerman MA, Clark GL, Abshire CM, Gentry KM, Miller KS, et al. New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol. 2018; 315:H1073–87.
40. Benarroch EE. Neuroscience for clinicians: basic processes, circuits, disease mechanisms, and therapeutic implications. New York: Oxford University Press;2021.
41. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017; 96:17–42.
42. Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005; 11:966–72.
43. Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci (Lond). 2017; 131:635–51.
44. Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, et al. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol. 2007; 33:410–9.
45. Muñoz Maniega S, Chappell FM, Valdés Hernández MC, Armitage PA, Makin SD, Heye AK, et al. Integrity of normal-appearing white matter: influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017; 37:644–56.
46. Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, et al. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010; 30:808–15.
47. Appelman AP, Exalto LG, van der Graaf Y, Biessels GJ, Mali WP, Geerlings MI. White matter lesions and brain atrophy: more than shared risk factors?: a systematic review. Cerebrovasc Dis. 2009; 28:227–42.
48. Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017; 317:1443–50.
49. Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol. 2017; 82:706–18.
50. Arvanitakis Z, Capuano AW. Author response: late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2019; 92:732.
51. Kester MI, van der Flier WM, Mandic G, Blankenstein MA, Scheltens P, Muller M. Joint effect of hypertension and APOE genotype on CSF biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2010; 20:1083–90.
52. Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016; 36:241–52.
53. Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. 2018; 14:148–56.
54. Austin SA, Katusic ZS. Loss of endothelial nitric oxide synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer’s disease. Circ Res. 2016; 119:1128–34.
55. Tian M, Zhu D, Xie W, Shi J. Central angiotensin II-induced Alzheimer-like tau phosphorylation in normal rat brains. FEBS Lett. 2012; 586:3737–45.
56. Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004; 44:29–34.
57. Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, et al. Impact of the treatment of isolated systolic hypertension on behavioral variables. Results from the systolic hypertension in the elderly program. Arch Intern Med. 1994; 154:2154–60.
58. Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998; 352:1347–51.
59. Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002; 162:2046–52.
60. Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003; 163:1069–75.
61. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008; 7:683–9.
62. Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, et al. Heart outcomes prevention evaluation: use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002; 324:699–702.
63. Aronow WS. Hypertension and cognitive impairment. Ann Transl Med. 2017; 5:259.
64. Gupta A, Perdomo S, Billinger S, Beddhu S, Burns J, Gronseth G. Treatment of hypertension reduces cognitive decline in older adults: a systematic review and meta-analysis. BMJ Open. 2020; 10:e038971.
65. Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020; 323:1934–44.
66. Mossello E, Pieraccioli M, Nesti N, Bulgaresi M, Lorenzi C, Caleri V, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015; 175:578–85.
67. Yang W, Luo H, Ma Y, Si S, Zhao H. Effects of antihypertensive drugs on cognitive function in elderly patients with hypertension: a review. Aging Dis. 2021; 12:841–51.
68. Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser MG, Guillon P, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) magnetic resonance imaging substudy. Circulation. 2005; 112:1644–50.
69. Chuang YF, Breitner JC, Chiu YL, Khachaturian A, Hayden K, Corcoran C, et al. Use of diuretics is associated with reduced risk of Alzheimer’s disease: the Cache County Study. Neurobiol Aging. 2014; 35:2429–35.
70. Peters R, Yasar S, Anderson CS, Andrews S, Antikainen R, Arima H, et al. Investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology. 2020; 94:e267–81.
71. Hanon O, Berrou JP, Negre-Pages L, Goch JH, Nádházi Z, Petrella R, et al. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive function And Systolic Blood Pressure Reduction open-label study. J Hypertens. 2008; 26:1642–50.
72. Radaideh GA, Choueiry P, Ismail A, Eid E, Berrou JP, Sedefdjian A, et al. Eprosartan-based hypertension therapy, systolic arterial blood pressure and cognitive function: analysis of Middle East data from the OSCAR study. Vasc Health Risk Manag. 2011; 7:491–5.
73. Kalra L. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2014; 82:1192.
74. Gelber RP, Ross GW, Petrovitch H, Masaki KH, Launer LJ, White LR. Antihypertensive medication use and risk of cognitive impairment: the Honolulu-Asia Aging Study. Neurology. 2013; 81:888–95.
75. Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015; 86:1299–306.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download