Abstract
The coronavirus disease 2019 (COVID-19) pandemic has been ongoing for more than 2 years. Many patients who recover from severe acute respiratory syndrome coronavirus 2 infection continue to have aftereffects such as dyspnea and fatigue, which may lead to functional decline. Therefore, the need for managing these symptoms using methods such as pulmonary rehabilitation (PR) has emerged. The purpose of this study was to report the effectiveness of PR in five patients with acute COVID-19. PR was performed in patients with persistent dyspnea and oxygen demand after COVID-19. All five patients were able to maintain an independent functional status before COVID-19. However, after acute COVID-19, they were unable to walk independently and needed assistance for activities of daily living due to dyspnea and fatigue. Therefore, they were referred to rehabilitation units, and PR was performed. The modified Medical Research Council dyspnea scale, maximal expiratory pressure (MEP), 6-minute walking test, forced vital capacity, and grip strength were assessed before and after PR, and the results were compared. After PR, the parameters improved, except for the MEP in one patient (patient 3) and the grip strength in another patient (patient 4). After PR, two out of five patients returned to work and the other three returned home. Therefore, we conclude that PR is necessary for patients with acute COVID-19 with activity limitations.
Since the outbreak of coronavirus disease 2019 (COVID-19) in 2019, pandemic infection has been ongoing for more than 2 years. It has been reported that many patients who recovered from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continue to have aftereffects even 3 to 4 weeks after the onset [1,2]. This manifestation is called post-acute COVID-19 syndrome [1,2]. Dyspnea, fatigue, chest pain, and cognitive disturbances are common symptoms of post-acute COVID-19 syndrome, which lead to decreased exercise capacity, functional decline, and thus, poor quality of life [3,4]. Since these aftereffects persist, there are some reports on the effectiveness and necessity of pulmonary rehabilitation (PR) [5]. Many patients were admitted to our medical center because of SARS-CoV-2 infection, and some experienced dyspnea and fatigue after acute treatment of SARS-CoV-2 infection. Therefore, we report a case series of PR performed in patients with acute COVID-19.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Seoul Medical Center (IRB No: 2022-08-002), and the requirement for informed consent from the patient was waived by the IRB.
Among COVID-19–infected patients, PR was conducted on five severe patients who needed oxygen therapy. The patients were diagnosed using polymerase chain reaction tests, and all of them were treated with steroids, remdesivir (VEKLURY, Gilead Sciences, Foster City, CA, USA), and oxygen therapy. Three participants were treated with high-flow oxygen therapy, one of which was placed on a mechanical ventilator for 21 days. The characteristics of the five patients are shown in Table 1.
According to the medical records, all patients were able to maintain an independent functional status before COVID-19. However, after acute COVID-19 and treatment, they were unable to walk independently and needed assistance in activities of daily living due to dyspnea and fatigue. Therefore, they were referred to the rehabilitation units of our hospital and PR was performed.
At the time of transfer, chest computed tomographies of each patient was taken and pulmonary fibrosis was found (Fig. 1). After their transfer, 17 to 21 sessions of PR were provided at the Department of Rehabilitation of Seoul Medical Center between March 2021 and February 2022. Four patients were hospitalized during the PR sessions, and the other patient was treated in an outpatient clinic. We used the following measurements to assess PR outcomes: modified Medical Research Council (mMRC) dyspnea scale, maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), 6-minute walking test (6 MWT), forced vital capacity (FVC), and grip strength. These indicators were evaluated and compared before and after the PR. The PR program comprised warm-up (5–10 minutes), main (30 minutes), and cool-down (5–10 minutes) exercises. The main exercises included flexibility exercise, inspiratory muscle strengthening, limb muscle strength training, and aerobic exercise. Aerobic exercise was mainly carried out as a treadmill exercise or cycle ergometer exercise. Aerobic exercise was performed at moderate intensity, targeting 60% of the maximal heart rate. The modified Borg scale (MBS) measures dyspnea to assess the patient’s tolerance. When the MBS score exceeded 3 points (i.e., moderate dyspnea), a short break was taken. During PR, oxygen was supplied if necessary to maintain an oxygen saturation above 90%. These all exercises were performed by a more experienced physical therapist. One patient (patient 3) did not require an oxygen supply during the first PR session. The remaining four patients initially required 1 to 5 L/min of oxygen. As the PR program progressed, the four patients who needed oxygen supply could taper out oxygen. Oxygen saturation was well maintained during the exercise without oxygen supply, and the patient’s dyspnea was tolerable. Subjective breathing difficulties were evaluated using mMRC. The results of the mMRC scale improved in all five patients after PR (Table 2 [6-8]). Among the three patients who started PR at mMRC level 4, two patients improved to level 1, and one patient improved to level 2. The other two patients started PR at mMRC level 3 and ended at level 2. MIP and MEP tests were performed to evaluate respiratory muscle strength. After PR, MIP and MEP results improved in all five patients, except for the MEP value of one patient (patient 3). The 6 MWT was performed to test endurance, and all five patients showed substantial improvement in distance. The FVC of all five patients also improved after PR. Grip strength was measured to determine the patients’ overall strength [9]. The grip strength of the four patients increased after PR. Finally, after PR, two out of five patients returned to work and the other three returned home.
COVID-19 pneumonia causes pulmonary fibrosis [10], and PR after pulmonary fibrosis helps reduce dyspnea, improve exercise capacity, and improve the quality of life [11]. Since exercise also works positively for the psychological, neurological, cardiovascular, respiratory, musculoskeletal, and immune systems [12], PR may also help the overall recovery of COVID-19 patients. Yet, the benefits of PR, including improvement in dyspnea, exercise capacity, and quality of life, are usually well described in chronic obstructive pulmonary disease (COPD) [13]. However, the effectiveness of PR for COVID-19 has not yet been clearly described. Currently, there is no panacea for COVID-19; therefore, the importance of adding PR to antiviral and anti-inflammatory drugs and symptom control is emerging.
In our report, we evaluated the mMRC, MEP/MIP, 6 MWT, FVC, and grip strength in five patients with acute COVID-19. mMRC scale assessed the degree of subjective dyspnea. MEP/MIP evaluated respiratory muscle performance [14,15], 6 MWT tested endurance and FVC estimated lung function after pulmonary fibrosis. Grip strength measured a patient’s overall strength, including muscle mass and physical function [9]. The parameters (mMRC, MEP/MIP, 6 MWT, FVC, and grip strength) improved, except for the MEP in one patient (patient 3) and grip strength in another patient (patient 4). For patient 3 whose MEP value did not improve after PR, the initial MEP result before PR was already 100% of the expected value. In other words, there may have been fewer opportunities for improvement. As the PR sessions progressed, dyspnea improved, as shown by improvement in the mMRC scale. The oxygen demand also decreased. As dyspnea is known to significantly impact the quality of life [3,16], it can be inferred that the quality of life of patients who received PR has improved. In addition, increased exercise capacity, indicated by improvements in the 6 MWT results, helped return to daily life. Even after the end of the PR sessions, the patients continued to visit the Department of Pulmonology and Rehabilitation Medicine for outpatient treatment. Later, when tracking the medical records, all five patients were found to have improved enough to experience no problems in their activities of daily living. Based on our findings, we suggest that PR may be helpful in treating COVID-19, as in previous COPD studies.
This study had some limitations. First, patients’ underlying conditions, such as preexisting respiratory disorder, comorbidity, premorbid activity level, or smoking history, were not considered. Second, the number of PR sessions was different for each patient. Therefore, it is difficult to propose a regular protocol for PR. Third, the sample size is relatively small. Additionally, there was only one intensive care unit case, and the disease level and severity level of the participant group was not consistently controlled. Fourth, there is a gender imbalance in the sample. Four of the five patients were men, and only one woman was included. Finally, this study can only be reported as a case series. Since COVID-19 has been a pandemic, it was difficult to conduct this study as a case-control study or randomized controlled trial due to the limited hospitalization period and bed use.
In this study, we performed PR testing in patients with acute COVID-19. After receiving PR, patients were able to return to their daily lives with improved function. Therefore, we conclude that intensive PR is necessary for patients with acute COVID-19 with activity limitations. In the future, large-scale studies comparing PR groups to non-PR groups and follow-up studies on the long-term prognosis of PR conducted in patients with acute COVID-19 will be helpful.
References
1. Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020; 324:2251–2.
2. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020; 370:m3026.
3. Montani D, Savale L, Noel N, Meyrignac O, Colle R, Gasnier M, et al. Post-acute COVID-19 syndrome. Eur Respir Rev. 2022; 31:210185.
4. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021; 27:601–15.
5. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- and American Thoracic Society-coordinated international task force. Eur Respir J. 2020; 56:2002197.
6. Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009; 54:1348–59.
7. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166:111–7.
8. Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011; 4:127.
9. Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015; 18:465–70.
10. Lechowicz K, Drożdżal S, Machaj F, Rosik J, Szostak B, Zegan-Barańska M, et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J Clin Med. 2020; 9:1917.
11. Lee AL, Hill CJ, McDonald CF, Holland AE. Pulmonary rehabilitation in individuals with non-cystic fibrosis bronchiectasis: a systematic review. Arch Phys Med Rehabil. 2017; 98:774–82.e1.
12. Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health. 2021; 18:5329.
13. Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006; 173:1390–413.
14. Kurtaiş Aytür Y, Köseoğlu BF, Özyemişçi Taşkıran Ö, Ordu-Gökkaya NK, Ünsal Delialioğlu S, Sonel Tur B, et al. Pulmonary rehabilitation principles in SARS-COV-2 infection (COVID-19): a guideline for the acute and subacute rehabilitation. Turk J Phys Med Rehabil. 2020; 66:104–20.
15. Severin R, Arena R, Lavie CJ, Bond S, Phillips SA. Respiratory muscle performance screening for infectious disease management following COVID-19: a highly pressurized situation. Am J Med. 2020; 133:1025–32.
16. Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, Nyirenda T, Friedman T, Gupta A, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020; 15:e0243882.
Fig. 1.
Chest computed tomography of patient 2. (A) Axial view and (B) coronal view (before pulmonary rehabilitation [PR]). Ground glass opacities and pulmonary fibrosis are present in both lungs. (C) Axial view and (D) coronal view (4-month follow-up after PR). Ground-glass opacities and pulmonary fibrosis of both lungs decreased compared to panels A and B.
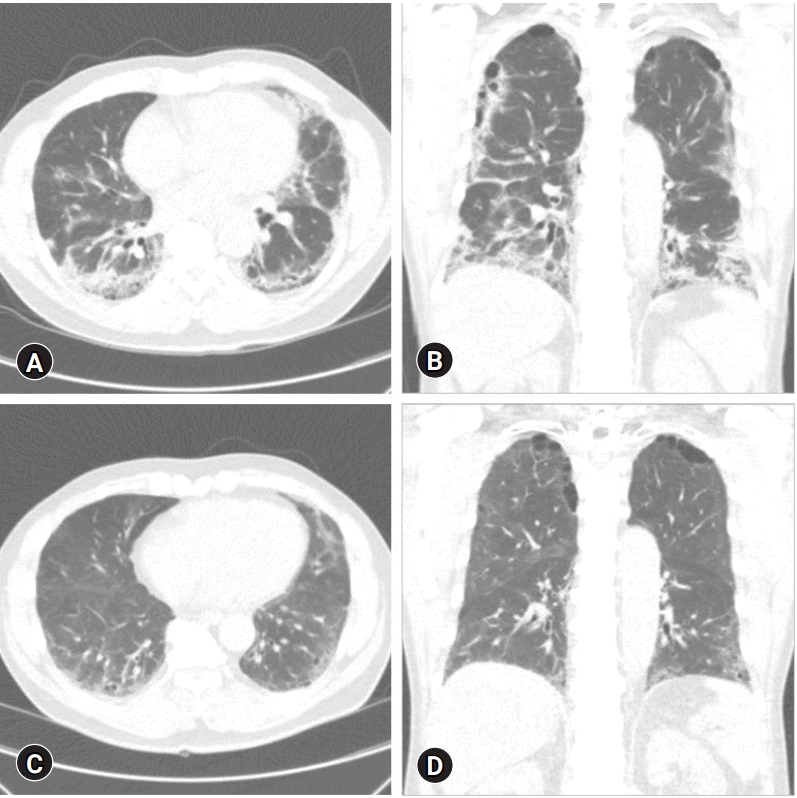
Table 1.
Patients’ characteristics
Table 2.
The change in various parameters after PR program
| Parameter |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before PR | After PR | Before PR | After PR | Before PR | After PR | Before PR | After PR | Before PR | After PR | |||
| mMRC dyspnea scale | 4 | 2 | 4 | 1 | 4 | 1 | 3 | 2 | 3 | 2 | ||
| MEP (cmH2O) [6] | 32 (42.7) | 49 (64.5) | 75 (60.0) | 86 (69.4) | 128 (100.0) | 114 (89.1) | 72 (59.5) | 103 (85.8) | 42 (34.7) | 83 (68.6) | ||
| MIP (cmH2O) [6] | 32 (61.5) | 53 (98.1) | 45 (47.9) | 69 (73.4) | 116 (119.6) | 118 (121.5) | 62 (66.0) | 76 (81.5) | 46 (52.1) | 86 (91.5) | ||
| FVC (L) | 1.59 (87.4) | 2.35 (90.0) | 2.81 (70.3) | 4.70 (71.1) | 3.81 (76.4) | 4.15 (80.4) | 2.76 (70.1) | 3.16 (80.2) | 3.12 (78.0) | 3.97 (89.6) | ||
| 6 MWT (m) [7] | 221 (49.7) | 265 (59.6) | 250 (46.5) | 475 (88.3) | 467 (78.4) | 540 (90.6) | 330 (62.5) | 440 (83.3) | 325 (62.7) | 450 (86.8) | ||
| Grip strength (kg) [8] | 15.5 (79.5) | 18.0 (92.3) | 38.8 (99.4) | 42.0 (107.7) | 34.5 (78.4) | 45.5 (103.3) | 20.0 (51.3) | 19.0 (48.7) | 14.0 (35.9) | 20.0 (51.2) | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download