Abstract
Background
Malnutrition and impaired immune responses significantly affect the clinical outcomes of patients with atherosclerotic stenosis. The Controlling Nutritional Status (CONUT) score has recently been utilized to evaluate perioperative immunonutritional status. This study aimed to evaluate the relationship between immunonutritional status, indexed by CONUT score, and postoperative complications in patients undergoing carotid endarterectomy (CEA).
Methods
We retrospectively evaluated 188 patients who underwent elective CEA between January 2010 and December 2019. The preoperative CONUT score was calculated as the sum of the serum albumin concentration, total cholesterol level, and total lymphocyte count. The primary outcome was postoperative complications within 30 days after CEA, including major adverse cardiovascular events, pulmonary complications, stroke, renal failure, sepsis, wounds, and gastrointestinal complications. Cox proportional hazards regression analysis was used to estimate the factors associated with postoperative complications during the 30-day follow-up period.
Results
Twenty-five patients (13.3%) had at least one major complication. The incidence of postoperative complications was identified more frequently in the high CONUT group (12 of 27, 44.4% vs. 13 of 161, 8.1%; p<0.001). Multivariate analyses showed that a high preoperative CONUT score was independently associated with 30-day postoperative complications (hazard ratio, 5.98; 95% confidence interval, 2.56–13.97; p<0.001).
Stroke is the second leading cause of mortality and adult disability [1]. Extracranial carotid artery disease accounts for 20% of all stroke cases. Previous studies have shown that carotid endarterectomy (CEA) is significantly effective in preventing cerebrovascular events in patients with high-grade stenosis of the carotid artery and, thus, is regarded as a ‘‘gold standard” treatment [2,3]. However, the benefits of CEA shown in previous studies may be limited at any time because of the development of perioperative complications that are influenced by patient-related factors. Indeed, patients undergoing CEA have a residual risk of future stroke or major cardiovascular events owing to the systemic nature of atherosclerosis [4]. The cause of this increased risk of postoperative complications in patients with CEA is unclear.
Malnutrition and immune responses are involved in the atherosclerotic process; their content is associated with the recurrence of stroke or cardiovascular events and is an important cause of postoperative complications in various surgeries [4,5]. Therefore, malnutrition-immune data may be necessary for categorizing risk and understanding the residual risk in patients undergoing CEA.
The Controlling Nutritional Status (CONUT) score can be determined from one immune marker (total lymphocyte count) and two metabolic parameters (serum albumin concentration and total cholesterol levels), which are representative indicators of immune defense, protein reserves, and lipid metabolism, respectively. A decrease in each parameter is assigned a higher score, with higher scores indicating poorer nutritional status. Compared to traditional screening tools, these CONUT score parameters consider the influence of non-nutritional factors. Although the CONUT score has recently been considered as a scoring system for predicting postoperative morbidity and evaluating nutritional status in various settings, including oncology therapy and cancer surgery, clinical data using the CONUT score to predict unfavorable prognosis in CEA patients are scarce [6,7]. Given that the CONUT score is a marker of nutritional and immune responses, this study aimed to confirm whether the CONUT score can predict postoperative complications independent of other known prognostic factors.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Ulsan University Hospital (IRB No: 2021-05-038), and the requirement for informed consent was waived.
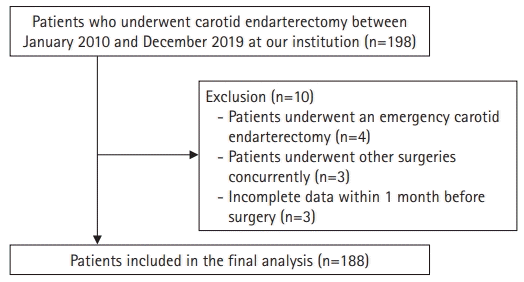
A total of 198 patients who underwent CEA between January 2010 and December 2019 were retrospectively evaluated. Patients who underwent an emergency CEA procedure (n=4), underwent other surgeries concurrently (n=3), or had incomplete or missing data (n=3) were excluded from this analysis. Thus, 188 patients were enrolled in the present study (Fig. 1). Clinical data, including demographic characteristics, comorbidities, postoperative complications, surgery side, degree of stenosis, and shunting, were reviewed using a computerized patient record system (Ulsan University Hospital Information of Clinical Ecosystem).
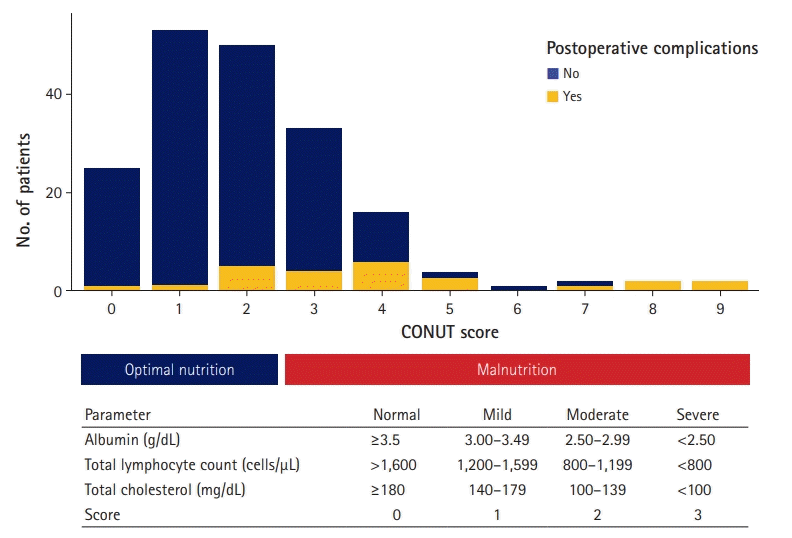
Blood samples were routinely collected within 1 week preceding CEA in all patients. The detection indices included white blood cells, lymphocyte counts, platelets, hemoglobin, C-reactive protein, albumin, blood urea nitrogen, serum creatinine, total cholesterol, total bilirubin, and uric acid. The immunonutritional status of each patient was evaluated based on the CONUT score. A score of >2 indicated malnutrition (Fig. 2) [8]. The CONUT score was calculated as the sum of three laboratory parameters: serum albumin level (g/dL), total lymphocyte count (cells/μL), and total cholesterol level (mg/dL), as summarized in Fig. 2.
The primary endpoint in our analysis was a composite of the main complications throughout the 30 days following CEA. The 30-day postoperative complications were selected according to the European Perioperative Clinical Outcome definitions [9] as follows: (1) major adverse cardiovascular events (e.g., malignant ventricular arrhythmia, myocardial infarction, and heart failure); (2) pulmonary complications; (3) stroke; (4) renal complications; (5) sepsis; (6) wound complications; (7) gastrointestinal complications; and (8) death. As secondary clinical outcomes, the duration of intensive care and hospitalization and readmission within 30 days after CEA were confirmed.
Descriptive variables are expressed as numbers (proportions), mean±standard deviation (SD), or medians (interquartile ranges [IQR]). Continuous variables were compared using the Student t-test, whereas the chi-square or Fisher exact test was used for categorical variables.
Cox proportional hazards regression analysis was used to estimate the factors associated with postoperative complications during the 30-day follow-up period. Unadjusted relationships between risk factors and 30-day postoperative complications were estimated using univariate Cox proportional hazards regression analysis and Kaplan-Meier curves. Only variables with a p-value of <0.05 on univariate analysis were incorporated into the backward multivariate analysis. Therefore, we adjusted for preoperative heart failure, clamping time, and serum albumin levels as potential confounders in the multivariate analysis. Statistical analyses were performed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) and R software ver. 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
The overall patient cohort had an average age of 67.14±8.04 years, and 79.3% were men. In total, 112 patients (59.6%) were symptomatic (Table 1). In our study, 32.4% of the patients were malnourished. Systolic blood pressure and diastolic blood pressure were 139.07±20.24 and 79.49±13.19 mmHg (mean±SD). Shunting was performed in 131 CEA procedures. All procedures were performed under general anesthesia. Detailed patient information is presented in Table 1.
The postoperative outcomes of the study are summarized in Table 2. At least 13.3% of the patients had one acute postoperative complication, and it was confirmed that the high CONUT group had a higher incidence of complications (12 of 27, 44.4% vs. 13 of 161, 8.1%; p<0.001) (Table 2). The CONUT score of the patients who underwent CEA and the distribution of postoperative complications based on the CONUT score are presented in Fig. 1. Concerning secondary clinical outcomes, it was confirmed that hospital stay was also extended in the group with a high CONUT score (median, 7 days [IQR, 6–17 days] vs. 6 days [IQR, 6–8 days], p=0.022) (Table 2).
Univariate and multivariate analyses were performed to identify prognostic factors for acute complications after surgery. The presence of heart failure (hazard ratio [HR], 8.43; 95% confidence interval [CI], 2.87–24.74; p<0.001), clamping time (HR, 0.97; 95% CI, 0.93–1.00; p=0.04), albumin (HR, 0.27; 95% CI, 0.14–0.53; p<0.001), and CONUT score (HR, 6.47; 95% CI, 2.95–14.20; p<0.001) were prognostic markers for postoperative complications. After adjusting for confounders, the CONUT score (HR, 5.98; 95% CI, 2.56–13.97; p<0.001) was an independent prognostic marker of acute postoperative complications (Table 3). Kaplan-Meier curves showed that the possibility of complications after CEA was significantly higher for patients with higher CONUT scores than for those with lower CONUT scores (p<0.001) (Fig. 3).
We evaluated the short-term prognostic value of the immunonutrition state using the CONUT score in patients who underwent CEA. Our findings showed a higher prevalence of postoperative complications in patients experiencing malnourishment than in those with normal nutrition.
Various nutritional screening tools are currently used to assess patient prognosis, as several studies have shown that malnutrition can lead to unfavorable outcomes [10]. Subjective Global Assessment and Mini Nutritional Assessment (MNA) are based on subjective data assessed by trained healthcare practitioners [11,12], whereas Prognostic Nutritional Index (PNI), Nutritional Risk Index (NRI), and CONUT scores are based on clinical or objective biochemical data [13,14]. To properly reflect the nutritional status of a patient, it would be more accurate to confirm both subjective and objective information. However, in a clinical setting, an objective, practical, and simple measurement method for primary nutritional screening may be more important. Several scoring systems, such as the PNI, NRI, and CONUT scores, have been proposed as reliable tools. Among the malnutrition scores, the CONUT score revealed the highest predictive ability for major adverse events in patients who underwent carotid artery stenting (CAS) [15]. The CONUT score was initially proposed by Ignacio de Ulíbarri et al. [16] as an easy and efficient nutritional screening tool for identifying malnutrition in hospitalized patients. A recent meta-analysis found that malnutrition calculated by the CONUT score was related to poor prognosis in surgical patients with hepatopancreatobiliary and gastrointestinal cancers [17]. Another study demonstrated an association between CONUT score and unfavorable clinical prognosis in hospitalized patients with various cardiovascular diseases [18]. Despite substantial evidence demonstrating the effectiveness of the CONUT score as an indicator of malnutrition for clinically unfavorable outcomes in various diseases, only one study has used the CONUT score as a prognostic indicator in patients undergoing CAS [15]. The results revealed that higher CONUT scores were associated with a clinically unfavorable prognosis in patients with CAS. In our study, CONUT score was positively associated with acute postoperative complications. However, more research is needed to assess which nutritional-immunological screening tools are the most practical and accurate in predicting clinically unfavorable prognosis after CEA.
Malnutrition accounts for a significant proportion of patients with stroke and neurological impairment, ranging from 6.1% to 62% [19]. According to the CONUT guidelines of our study, 32.4% of patients undergoing CEA were classified as malnourished. Thus, it is essential to elucidate how nutritional status affects acute postoperative complications in patients undergoing CEA. However, the exact pathophysiological mechanism underlying the association between CONUT score and increased postoperative morbidity remains unknown. Indeed, the mechanism of the relationship between CONUT score and increased risk of postoperative complications is considered multifactorial. Other investigators have hypothesized that malnutrition increases major postoperative complications owing to alterations in protein metabolism or decreased physiological reserves to cope with acute surgical stress [20]. In other words, malnutrition itself may lead to a poor prognosis after surgery due to multifactorial consequences such as decreased protein synthesis, increased inflammatory response, and changes in immune function. Interestingly, immunonutritional status was readily assessed using the CONUT score based on serum albumin, total cholesterol, and lymphocyte count because a decrease in the response of each variable with acute disease or acute surgical stress may reflect a low immune nutritional status. Of the three CONUT components, serum albumin level was the most critical parameter. It is generally considered a biomarker of nutritional and systemic inflammatory status. Therefore, hypoalbuminemia may contribute to the progression and development of atherosclerosis [21].
Furthermore, hypoalbuminemia is associated with reduced antioxidant and antiplatelet aggregation activities, which result in increased central mediators of cardiovascular stenosis and increased oxidative stress and blood viscosity, leading to cardiovascular complications. In our study, these mechanisms may have influenced the association between malnutrition and cardiovascular complications. Moreover, lymphocytopenia affected the postoperative complications in patients who underwent CEA in our study. Indeed, low lymphocyte counts may indicate impaired host immunity and poor nutrient intake [22]. Lymphocytopenia has also been studied in association with malnutrition and systemic inflammatory conditions. It has been speculated that specific pathophysiological courses may cause an acute increase in stress-related steroid levels, leading to a decrease in lymphocyte count [23]. In addition, low cholesterol levels may indicate progressive disease and systemic inflammatory activation [24]. Therefore, the CONUT score assesses the immunonutritional status of patients with caloric depletion, reduced protein reserves, and impaired immune defenses, which may serve as incremental values in predicting postoperative complications.
Our results suggest that screening the nutritional status of patients admitted for CEA can identify those at a higher risk of postoperative complications. Additionally, identifying malnutrition in patients undergoing CEA may lead to interventions for secondary prevention such as oral nutritional supplements, dietary counseling, food/fluid enrichment, and educational interventions [25]. There are many malnutrition screening tools, but there are still no standard guidelines for treating patients undergoing CEA. The management of atherosclerotic carotid stenosis is still under development, and the prognostic capabilities of the nutritional index have been found to be valuable and practical in several studies [26]. Therefore, the CONUT score, an easy and objective scoring system, may be selected as a valuable nutritional index for predicting unfavorable clinical outcomes in patients undergoing CEA, in addition to traditional parameters.
Our study has some limitations. First, this was a single-center retrospective observational study with a relatively small patient cohort. Second, our study assessed CONUT scores only on admission and did not evaluate CONUT scores after hospital discharge. Third, we only evaluated CONUT scores as indicators of malnutrition. Due to the retrospective nature of our study, other nutritional indicators, such as the Maastricht Index, MNA Short-Form scale, and NRI, were not used. Fourth, considering the retrospective nature of this study, we verified the post hoc power of the sample size. The incidence of acute postoperative complications after CEA in patients enrolled in this study was 13%. For the sample size of 188 people, when an alpha error of 0.05 was considered, only 85% of the post-test power was confirmed. These limitations require further investigation to validate the results. Large multicenter and prospective studies with larger numbers of patients are needed.
In conclusion, this study revealed that malnutrition assessment using the CONUT score can identify patients undergoing CEA who are at elevated risk for postoperative complications. Evaluation of immunonutritional status by CONUT score may help stratify the risk of acute postoperative complications and encourage improvement in nutritional status.
Notes
Author contributions
Conceptualization, Formal analysis: HWS, GY, SJL, JO; Investigation: HWS, GY, SJL; Data curation: HWS, SJL; Methodology: GY, SJL; Project administration, Validation: JO; Visualization, Supervision: HWS, JO; Software: SJL; Writing-original draft: HWS, JO; Writing-review & editing: HWS, JO.
References
1. French BR, Boddepalli RS, Govindarajan R. Acute ischemic stroke: current status and future directions. Mo Med. 2016; 113:480–6.
2. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–8.
3. European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991; 337:1235–43.
4. Timmerman N, Waissi F, Dekker M, van de Pol QY, van Bennekom J, Schoneveld A, et al. Pre-operative plasma extracellular vesicle proteins are associated with a high risk of long term secondary major cardiovascular events in patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg. 2021; 62:705–15.
5. Jin H, Zhu K, Wang W. The predictive values of pretreatment controlling nutritional status (CONUT) score in estimating short- and long-term outcomes for patients with gastric cancer treated with neoadjuvant chemotherapy and curative gastrectomy. J Gastric Cancer. 2021; 21:155–68.
6. Wang A, He Z, Cong P, Qu Y, Hu T, Cai Y, et al. Controlling nutritional status (CONUT) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to NLR and PNI: a single-center retrospective study. Front Oncol. 2021; 10:593452.
7. Müller L, Hahn F, Mähringer-Kunz A, Stoehr F, Gairing SJ, Foerster F, et al. Immunonutritive scoring in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: Prognostic Nutritional Index or Controlling Nutritional Status score? Front Oncol. 2021; 11:696183.
8. Kato T, Yaku H, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, et al. Association with controlling nutritional status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep. 2020; 10:3320.
9. Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015; 32:88–105.
10. Poulia KA, Yannakoulia M, Karageorgou D, Gamaletsou M, Panagiotakos DB, Sipsas NV, et al. Evaluation of the efficacy of six nutritional screening tools to predict malnutrition in the elderly. Clin Nutr. 2012; 31:378–85.
11. Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999; 15:116–22.
12. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987; 11:8–13.
13. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005; 82:777–83.
14. Wang ZJ, Zhou YJ, Galper BZ, Gao F, Yeh RW, Mauri L. Association of body mass index with mortality and cardiovascular events for patients with coronary artery disease: a systematic review and meta-analysis. Heart. 2015; 101:1631–8.
15. Çakmak EÖ, Öcal L, Erdoğan E, Cerşit S, Efe SÇ, Karagöz A, et al. Prognostic value of 3 nutritional screening tools to predict 30-day outcome in patients undergoing carotid artery stenting. Angiology. 2022; 73:225–33.
16. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005; 20:38–45.
17. Takagi K, Domagala P, Polak WG, Buettner S, Ijzermans JN. The controlling nutritional status score and postoperative complication risk in gastrointestinal and hepatopancreatobiliary surgical oncology: a systematic review and meta-analysis. Ann Nutr Metab. 2019; 74:303–12.
18. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. 2020; 76:828–40.
19. Foley NC, Salter KL, Robertson J, Teasell RW, Woodbury MG. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke. 2009; 40:e66–74.
20. Steenhagen E. Preoperative nutritional optimization of esophageal cancer patients. J Thorac Dis. 2019; 11(Suppl 5):S645–53.
21. Schillinger M, Exner M, Mlekusch W, Amighi J, Sabeti S, Schlager O, et al. Serum albumin predicts cardiac adverse events in patients with advanced atherosclerosis: interrelation with traditional cardiovascular risk factors. Thromb Haemost. 2004; 91:610–8.
22. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005; 45:1638–43.
23. Cereda E, Pusani C, Limonta D, Vanotti A. The association of Geriatric Nutritional Risk Index and total lymphocyte count with short-term nutrition-related complications in institutionalised elderly. J Am Coll Nutr. 2008; 27:406–13.
24. Gomes F, Emery PW, Weekes CE. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J Stroke Cerebrovasc Dis. 2016; 25:799–806.
25. Bandayrel K, Wong S. Systematic literature review of randomized control trials assessing the effectiveness of nutrition interventions in community-dwelling older adults. J Nutr Educ Behav. 2011; 43:251–62.
26. Paraskevas KI, Mikhailidis DP, Veith FJ, Spence JD. Definition of best medical treatment in asymptomatic and symptomatic carotid artery stenosis. Angiology. 2016; 67:411–9.
Fig. 3.
Kaplan-Meier curves for the development of acute postoperative complications between different Controlling Nutritional Status (CONUT) scores.
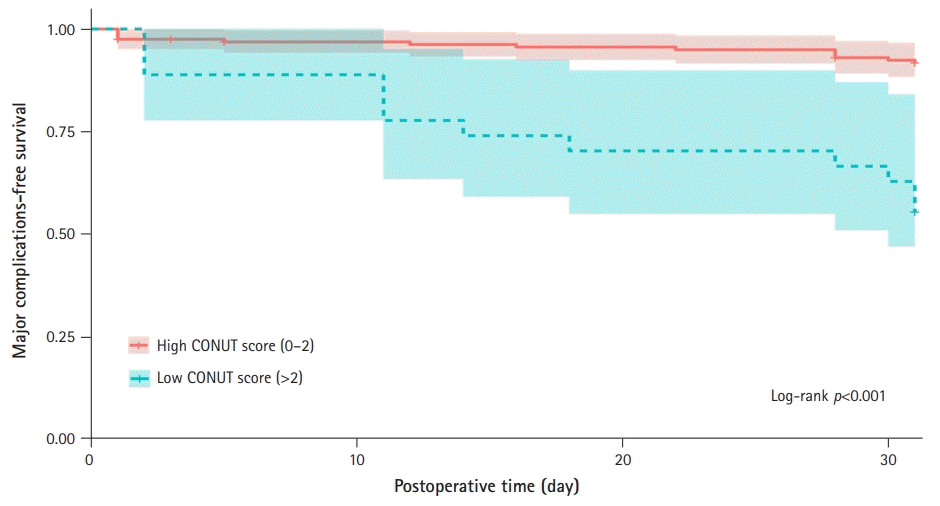
Table 1.
Preoperative clinical characteristics of study participants
Table 2.
Postoperative outcomes for CONUT groups
| Variable | Total (n=188) | Low CONUT (n=161) | High CONUT (n=27) |
|---|---|---|---|
| Primary outcome | |||
| Postoperative complications | 25 (13.3) | 13 (8.1) | 12 (44.4)a) |
| Major adverse cardiovascular events | 7 | 2 | 5 |
| Pulmonary complications | 4 | 2 | 2 |
| Stroke | 9 | 6 | 3 |
| Renal complications | 1 | 1 | 0 |
| Sepsis | 1 | 0 | 1 |
| Wound complications | 1 | 1 | 0 |
| Gastrointestinal complications | 1 | 1 | 0 |
| Death | 1 | 0 | 1 |
| Secondary outcome | |||
| Intensive care unit stay (hr) | 33.7±84.3 | 34.2±90.8 | 30.9±20.9 |
| Hospital stay (day) | 7 (6–8) | 6 (6–8) | 7 (6–17)a) |
| 30-Day readmission | 2 (1.1) | 1 (0.6) | 1 (3.7) |
Table 3.
Predictors associated with acute postoperative complications in patients undergoing carotid endarterectomy
| Variable |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Demographics | ||||
| Female sex | 0.96 (0.36–2.56) | 0.94 | ||
| Age | 1.03 (0.97–1.08) | 0.35 | ||
| Body mass index | 0.85 (0.74–0.98) | 0.44 | ||
| Diabetes mellitus | 0.67 (0.29–1.56) | 0.35 | ||
| Smoker | 0.98 (0.94–1.02) | 0.45 | ||
| Hypertension | 0.56 (0.25–1.23) | 0.15 | ||
| Dyslipidemia | 0.40 (0.00–6.57) | 0.22 | ||
| Heart failure | 8.43 (2.87–24.74) | <0.001 | ||
| Previous ischemic heart disease | 1.07 (0.37–3.13) | 0.90 | ||
| Atrial fibrillation | 1.78 (0.61–5.17) | 0.29 | ||
| Baseline heart rate | 1.01 (0.98–1.04) | 0.60 | ||
| Baseline systolic blood pressure | 1.02 (0.99–1.06) | 0.15 | ||
| Baseline diastolic blood pressure | 1.02 (1.00–1.04) | 0.09 | ||
| Symptomatic lesion | 2.29 (0.91–5.73) | 0.08 | ||
| Antiplatelet use | 1.20 (0.16–8.88) | 0.86 | ||
| Antiplatelet, >1 | 1.17 (0.51–2.64) | 0.71 | ||
| Intraoperative variable | ||||
| Shunt use | 0.64 (0.29–1.43) | 0.28 | ||
| Operation time | 1.00 (0.99–1.00) | 0.54 | ||
| Clamping time | 0.97 (0.93–1.00) | 0.04 | ||
| Operation side, left | 1.03 (0.47–2.27) | 0.93 | ||
| Ipsilateral stenosis, >70% | 1.16 (0.35–3.88) | 0.81 | ||
| Hematologic biomarker and nutrition index | ||||
| Hematocrit | 0.96 (0.89–1.03) | 0.23 | ||
| Albumin | 0.27 (0.14–0.53) | <0.001 | ||
| Total cholesterol | 0.99 (0.98–1.00) | 0.09 | ||
| Creatinine | 0.82 (0.35–1.94) | 0.66 | ||
| Uric acid | 0.96 (0.74–1.23) | 0.71 | ||
| Triglyceride | 1.00 (0.99–1.01) | 0.81 | ||
| High-density lipoprotein | 0.99 (0.95–1.03) | 0.53 | ||
| Low-density lipoprotein | 1.00 (0.99–1.01) | 0.60 | ||
| CONUT score, >2 | 6.47 (2.95–14.20) | <0.001 | 5.98 (2.56–13.97) | <0.001a) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download