Abstract
Background
The purpose of this study was to investigate whether postoperative cystography findings can predict early and long-term recovery from incontinence after radical prostatectomy (RP), compared with the other cystography parameters.
Methods
I retrospectively reviewed 118 patients who underwent robot-assisted RP (RARP) for localized prostate cancer at single institution between January 2016 and April 2021. One hundred and seven patients were included in the study. Postoperative cystography was routinely performed 7 days after surgery. The bladder neck to pubic symphysis ratio, vesicourethral angle, and bladder neck anteroposterior length (BNAP) ratio (the bladder neck-posterior margin distances divided by the anteroposterior lengths) were evaluated. Continence was defined as cessation of pad use. The association between these variables and urinary incontinence was also analyzed.
Results
The urinary incontinence recovery rates 1, 3, 6, and 12 months after RARP were 43.92%, 66.35%, 87.85%, and 97.19%, respectively. Multivariate logistic regression analysis demonstrated that a lower BNAP ratio and wider vesicourethral angle were significantly associated with continence restoration at 1, 3, and 6 months after surgery. In addition, in terms of days of pad usage, lower BNAP ratio, wider vesicourethral angle, and bladder neck preservation were significantly associated with recovery from urinary incontinence within 12 months as assessed by Cox proportional hazard analysis.
Radical prostatectomy (RP) is the definitive treatment for localized prostate cancer. However, post-prostatectomy incontinence (PPI) is a significant surgical complication after RP that can influence a patient’s quality of life. The major causes of PPI include damage to the urethral sphincter, bladder instability, and destruction of pelvic support [1-3]. Despite the fact that there are several surgical techniques for preventing PPI, such as nerve-sparing techniques, bladder neck preservation, and posterior reconstruction [4-6], PPI remains a major obstacle.
Recent studies have indicated that perioperative imaging can be used to predict PPI. Mendoza et al. [7] reported that longer urethral lengths on preoperative magnetic resonance imaging (MRI) were associated with faster continence recovery at all postoperative time points. Coakley et al. [8] also found that a longer membranous urethra on endorectal MRI before RP was significantly associated with a more rapid return to continence [8]. However, immediate assessment of postoperative urethral length may be more important than preoperative urethral length in predicting PPI because urethral length can be shorter after surgery.
In contrast, several studies have reported that postoperative cystography, a simple method that is routinely performed to check for urinary leakage after surgery, can predict PPI. Olgin et al. [9] reported that the post-prostatectomy bladder neck location on cystography correlates with continence rates and predicts patients at risk of prolonged incontinence [9]. Sugi et al. [10] also demonstrated that a narrow vesicourethral angle measured by cystography is a useful predictor of PPI.
Although these studies have shown promising results, the efficacy of postoperative cystography in predicting early recovery from PPI is controversial because there are no systematic reviews or randomized controlled trials on the efficacy of postoperative cystography. In addition, previous studies on the efficacy of postoperative cystography assessed only two-dimensional aspects, such as the coronal view. Therefore, I focused on the three-dimensional aspect as a predictor of early recovery from PPI by using not only the coronal view but also the sagittal view on cystography. In this study, I investigated the efficacy of a new cystography parameter (i.e., the sagittal view) to predict early and long-term recovery from incontinence after RP, comparing it with a well-known previous cystography parameter (i.e., the coronal view) on postoperative cystography.
Ethical statements: The study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2020-04-061-001), which waived the need for informed consent owing to the retrospective design of the study.
I retrospectively reviewed 118 patients who underwent robot-assisted RP (RARP) for localized prostate cancer at single institution between January 2016 and April 2021. One hundred and seven patients were included in the study. The exclusion criteria were as follows: radiation therapy within 1 year, urine leakage from the anastomosis during cystography, previous urethral or prostate surgery, and no nerve-sparing procedure.
All patients underwent RARP performed by an experienced surgeon at a single institution. The surgeon performed the procedures as follows: bladder neck reconstruction (if needed), posterior reconstruction, and vesicourethral anastomosis. All patients underwent posterior reconstruction using the Rocco stitch [11]. Vesicourethral anastomosis was performed using a bidirectional barbed (V-Loc 90; Medtronic, Minneapolis, MN, USA) running suture in all patients. Unilateral or bilateral neurovascular bundle (NVB) sparing procedures were performed in all patients according to their clinical stage.
Cystography was routinely performed postoperatively 7 days before urethral catheter removal. The bladder was filled with 150-mL saline solution with contrast medium, and front, semilateral, and lateral-view images were acquired. If vesicourethral anastomosis leakage was observed, the cystography was repeated after 7 days.
I calculated the bladder neck to pubic symphysis (BNPS) ratio (defined as the bladder neck-pubis symphysis distance divided by the total pubis symphysis height) (Fig. 1A) and vesicourethral angle (measured as the angle of the bladder neck relative to the bilateral margin over the pelvic inlet) (Fig. 1B). I also calculated the anteroposterior length of the bladder and distance from the most posterior margin of the bladder to the bladder neck using lateral-view cystography. To control for potential differences in magnification by cystography, the bladder neck-posterior margin distances were divided by the anteroposterior lengths, called the bladder neck anteroposterior length (BNAP) ratio (Fig. 1C). All cystography parameters were analyzed by a single urologist who was blinded to the continence results.
All 107 patients were routinely followed up at 1, 3, 6, and 12 months after RARP. The patients reported daily pad use and the last date of pad usage at each visit. Continence was defined as the cessation of pad use.
Univariate analysis was performed using the Student t-test for continuous variables and the chi-square test for categorical variables at 1, 3, 6, and 12 months after surgery. Multivariate logistic regression analysis was used to confirm independent predictive factors for urinary incontinence. According to the time of pad use, the Cox proportional hazard model was used to identify predictors of recovery from urinary incontinence. In addition, cut-off values for independent factors of urinary incontinence on cystography parameters were determined using receiver operating characteristic (ROC) curves. All analyses were performed using IBM SPSS ver. 19.0 (IBM Corp. Armonk, NY, USA). Statistical significance was set at p<0.05.
The patient characteristics are shown in Table 1. The recovery rates for urinary incontinence at 1, 3, 6, and 12 months after surgery were 43.92%, 66.35%, 87.85%, and 97.19%, respectively. The cumulative recovery curve for urinary incontinence is shown in Fig. 2.
Univariate analysis for predictive factors of continence recovery at 1, 3, 6, and 12 months after surgery is shown in Table 2. In the univariate analysis, bladder neck preservation was significantly associated with early continence restoration 1 month after RARP. Lower BNAP ratio and wider vesicourethral angle were statistically significant predictors of PPI at 1, 3, 6, and 12 months after RARP. However, a lower BNPS ratio was associated with continence recovery at 1, 3, and 6 months after surgery. Multivariate logistic regression analysis demonstrated that a lower BNAP ratio and wider vesicourethral angle were significantly associated with continence restoration at 1, 3, and 6 months after surgery (Table 3). The Cox-Snell R-squared values were 0.577, 0.579, and 0.375 at 1, 3, and 6 months after surgery, respectively. Meanwhile, in terms of the days of pad usage, lower BNAP ratio, wider vesicourethral angle, and bladder neck preservation were significantly associated with recovery from urinary incontinence within 12 months in the Cox proportional hazard analysis (Table 4).
In the ROC curve analysis, the BNAP ratio and vesicourethral angle were superior to the bladder neck location in terms of sensitivity, specificity, and Youden’s index (Table 5). In addition, the areas under the curve of the BNAP ratio and vesicourethral angle 1 month after surgery were higher than those of the BNPS ratio (0.898, 0.918, and 0.650, respectively). The optimal cut-off values for the BNAP ratio, vesicourethral angle, and BNPS ratio 1 month after surgery were 0.224, 108.5, and 0.584, respectively.
As early recovery from PPI is still an important factor associated with the patients’ quality of life, many perioperative parameters and surgical techniques related to early recovery from urinary incontinence after RARP have been reported [8-11]. In particular, immediate postoperative imaging such as MRI or cystography may facilitate PPI prognosis. However, immediate postoperative MRI is expensive for assessing only the early recovery from PPI in real practice. Thus, many studies have reported the efficacy of postoperative cystography as a predictive factor for early recovery from PPI [9,10].
In postoperative cystography, the location of the bladder neck has been associated with early recovery from PPI in several studies. Jeong et al. [12] reported a correlation between bladder neck location and early recovery from PPI in a large cohort. They concluded that a higher location of the bladder neck leads to a higher rate of early recovery from PPI. Olgin et al. [9] and Kageyama et al. [13] also reported that a lower bladder neck position may predict prolonged urinary incontinence after RARP. They argued that damage to the levator ani muscle, pubourethral ligament, puboprostatic ligament, and median fibrous raphe, which suspend and support the bladder, prostate, and urethra, may lead to a decrease in the bladder neck location and change in the vesicourethral angle. They hypothesized that this contributes to normal continence impairment. However, there was no statistically significant difference between the location of the bladder neck and recovery of urinary incontinence at any point in this study. I hypothesized that, despite the low bladder neck location, there would not be a change in force transmission during the stress state if the bladder neck angle was still wide.
Several recent studies have reported that a wide bladder neck angle on postoperative cystography is a predictive factor for PPI recovery. Sugi et al. [10] reported that a narrow vesical angle is significantly associated with urinary incontinence at 1 month and 1 year after RARP. Shao et al. [14] also suggested that a shaped bladder neck angle was a significant predictor of urinary incontinence 6 months after RARP. However, there were several limitations in previous studies regarding the vesicourethral angle. For example, in Sugi et al. [10], several surgeons performed RARP. In this study, patients with a wide bladder neck angle on postoperative cystography showed early restoration of urinary incontinence after RARP, as the clinical variables were completely controlled. At the onset of the normal voiding phase in men, the bladder neck is opened and the posterior vesicourethral angle changes sharply, with striated urethral sphincter relaxation and anterior fibromuscular stroma contraction [15]. I anticipated that as the urethral stump and bladder neck were more tensioned when vesicourethral anastomosis was performed, the shape of the bladder would be more prolate, and the bladder neck angle would be narrow. Therefore, a narrow bladder neck angle is associated with restoration of urinary incontinence.
A unique aspect of the present study was that the BNAP ratio was associated with early continence recovery after RARP. In previous studies, postoperative cystography parameters were only measured in the coronal view of cystography [9,10,12-14,16]. However, I anticipated that the force transmission of urine from the bladder neck to the urethra could be three-dimensional rather than two-dimensional. Therefore, I focused on the sagittal view of postoperative cystography, which is an important parameter related to PPI recovery.
Therefore, I must consider why a lower BNAP ratio is associated with early continence recovery. When abdominal pressure increases, stable urethral support is an important mechanism for controlling urinary incontinence [17]. Because the mechanism of urinary incontinence after RP is similar to that of stress urinary incontinence in women, posterior reconstruction of the rhabdosphincter in RARP can be helpful for early continence recovery [11]. I hypothesized that urethral support would be more stable if the bladder neck was located more posteriorly after posterior reconstruction. In addition, it can be anticipated that as the bladder neck becomes more posterior, the vesicourethral angle may narrow. Therefore, I anticipated that a low BNAP ratio would influence early recovery from PPI.
The NVB-sparing procedure has been strongly associated with early continence recovery after RARP in many studies [18]. Therefore, I analyzed the differences in postoperative cystography features according to the state of NVB sparing. In the current study, there was no significant difference in the BNPS ratio, vesicourethral angle, and BNAP ratio between the unilateral and bilateral NVB-sparing procedures. Therefore, the vesicourethral angle and BNAP ratio could be predictive factors for early continence recovery after RARP regardless of NVB sparing.
By analyzing the ROC curve, I evaluated the statistical significance of the BNAP ratio compared to other cystography parameters such as bladder neck location and vesicourethral angle. In the ROC curve, the BNAP ratio and vesicourethral angle values were superior to those of the bladder neck location in terms of sensitivity and specificity. Therefore, the BNAP ratio can effectively predict early recovery from PPI. In addition, the combination of these parameters could predict more outcomes than each parameter alone, although I only evaluated the efficacy of each postoperative cystography parameter.
This study had several limitations. First, this was a retrospective study conducted at a single center. Second, I did not use the 1-hour pad test to assess the degree of incontinence on each follow-up day. Finally, preoperative cystography to measure baseline parameters was not performed in this study. Nevertheless, this is the first study to assess the efficacy of predictive parameters for PPI recovery using three-dimensional aspects of postoperative cystography. In addition, the current study presents a new concept for predicting continence recovery after RARP using postoperative cystography.
In conclusion, this study demonstrated that the BNAP ratio and vesicourethral angle are associated with the prediction of early recovery from urinary incontinence after RARP. Sagittal and coronal cystography views may help predict early restoration of urinary continence. In addition, the use of a combination of cystography parameters may be more helpful than the use of any single parameter alone.
References
1. Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997; 157:233–6.
2. Leach GE, Trockman B, Wong A, Hamilton J, Haab F, Zimmern PE. Post-prostatectomy incontinence: urodynamic findings and treatment outcomes. J Urol. 1996; 155:1256–9.
3. Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010; 57:179–92.
4. Srivastava A, Chopra S, Pham A, Sooriakumaran P, Durand M, Chughtai B, et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2013; 63:438–44.
5. Freire MP, Weinberg AC, Lei Y, Soukup JR, Lipsitz SR, Prasad SM, et al. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol. 2009; 56:972–80.
6. Rocco F, Carmignani L, Acquati P, Gadda F, Dell'Orto P, Rocco B, et al. Early continence recovery after open radical prostatectomy with restoration of the posterior aspect of the rhabdosphincter. Eur Urol. 2007; 52:376–83.
7. Mendoza PJ, Stern JM, Li AY, Jaffe W, Kovell R, Nguyen M, et al. Pelvic anatomy on preoperative magnetic resonance imaging can predict early continence after robot-assisted radical prostatectomy. J Endourol. 2011; 25:51–5.
8. Coakley FV, Eberhardt S, Kattan MW, Wei DC, Scardino PT, Hricak H. Urinary continence after radical retropubic prostatectomy: relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. J Urol. 2002; 168:1032–5.
9. Olgin G, Alsyouf M, Han D, Li R, Lightfoot M, Smith D, et al. Postoperative cystogram findings predict incontinence following robot-assisted radical prostatectomy. J Endourol. 2014; 28:1460–3.
10. Sugi M, Kinoshita H, Yoshida T, Taniguchi H, Mishima T, Yoshida K, et al. The narrow vesicourethral angle measured on postoperative cystography can predict urinary incontinence after robot-assisted laparoscopic radical prostatectomy. Scand J Urol. 2018; 52:151–6.
11. Rocco B, Gregori A, Stener S, Santoro L, Bozzola A, Galli S, et al. Posterior reconstruction of the rhabdosphincter allows a rapid recovery of continence after transperitoneal videolaparoscopic radical prostatectomy. Eur Urol. 2007; 51:996–1003.
12. Jeong SJ, Yi J, Chung MS, Kim DS, Lee WK, Park H, et al. Early recovery of urinary continence after radical prostatectomy: correlation with vesico-urethral anastomosis location in the pelvic cavity measured by postoperative cystography. Int J Urol. 2011; 18:444–51.
13. Kageyama S, Yoshida T, Nagasawa M, Kubota S, Tomita K, Kobayashi K, et al. The location of the bladder neck in postoperative cystography predicts continence convalescence after radical prostatectomy. BMC Urol. 2018; 18:52.
14. Shao IH, Chou CY, Huang CC, Lin CF, Chang YH, Tseng HJ, et al. A specific cystography pattern can predict postprostatectomy incontinence. Ann Surg Oncol. 2015; 22(Suppl 3):S1580–6.
15. Nishio K, Soh S, Syukuya T, Sato R, Sadaoka Y, Iwahata T, et al. Role of male pelvic floor muscles and anterior fibromuscular stroma in males on α(1)-blocker treatment: a magnetic resonance imaging study. Int J Urol. 2014; 21:724–7.
16. Kwon SY. Association between cystographic anastomotic urinary leakage following retropubic radical prostatectomy and early urinary incontinence. Yeungnam Univ J Med. 2021; 38:142–7.
17. Petros PE, Woodman PJ. The integral theory of continence. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:35–40.
18. Reeves F, Preece P, Kapoor J, Everaerts W, Murphy DG, Corcoran NM, et al. Preservation of the neurovascular bundles is associated with improved time to continence after radical prostatectomy but not long-term continence rates: results of a systematic review and meta-analysis. Eur Urol. 2015; 68:692–704.
Fig. 1.
Postoperative cystography parameters. (A) Bladder neck to pubic symphysis, (B) vesicourethral angle, and (C) bladder neck anteroposterior (BNAP) length. BNPS, bladder neck to pubic symphysis.
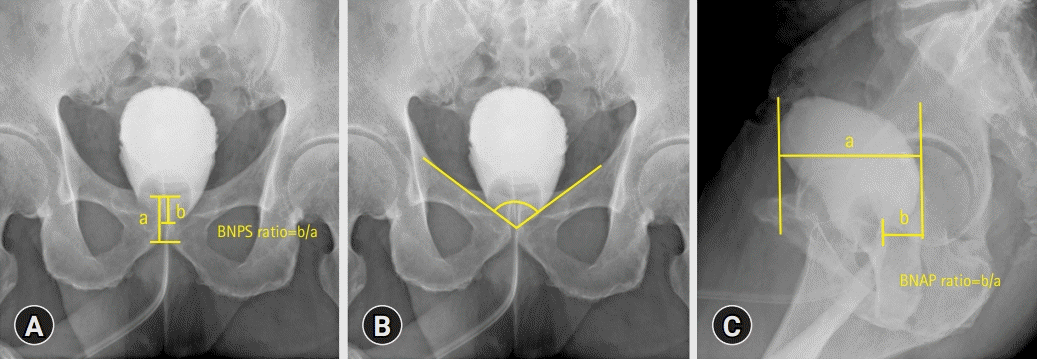
Fig. 2.
Cumulative recovery curve from urinary incontinence after robot-assisted radical prostatectomy.
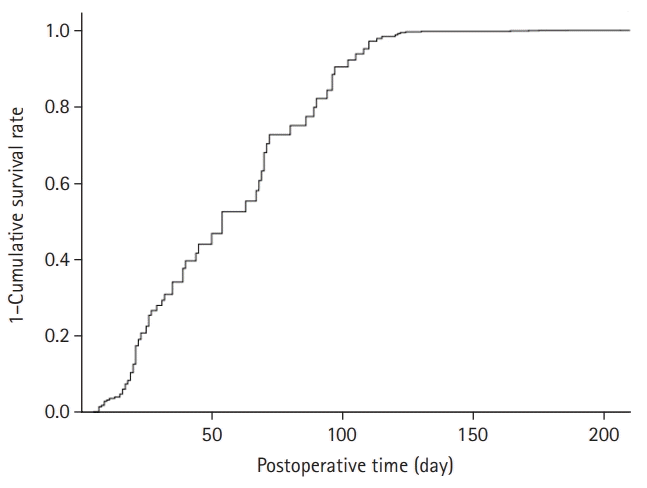
Table 1.
Characteristics of all patients
Table 2.
Univariate analysis for predictive factors of PPI at 1, 3, 6, and 12 months after surgery
Table 3.
Multivariate logistic regression analysis for predictive factors of PPI at 1, 3, and 6 months after surgery
Table 4.
Cox proportional hazard analysis for predictive factors of PPI within 12 months after surgery
Table 5.
Comparison of the cystography parameters for predictive factors of continence recovery 1 month after surgery in ROC curve analysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download