Abstract
Purpose
Whether administering chemotherapy followed by tamoxifen plus a gonadotropin-releasing hormone (GnRH) agonist to treat patients with lower-risk hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer provides a greater benefit than administering tamoxifen plus GnRH agonist alone remains unclear. This study aimed to compare the outcomes of propensity score-matched (PSM) patients who underwent these 2 types of treatment plans.
Methods
This retrospective study included patients treated at our institution between 2009 and 2019. Eligible patients had HR-positive, HER2-negative, invasive breast cancer who had undergone surgery. There were 579 patients with HR-positive, HER2-negative breast cancer who were treated with a GnRH agonist and tamoxifen; patients with pathologic N2 and those who received neoadjuvant chemotherapy were excluded. After 1:1 PSM of patients who underwent GnRH agonist treatment and tamoxifen with versus without chemotherapy, 122 patients from these 2 groups were analyzed. Survival rates were calculated using the Kaplan-Meier method and compared via the log-rank test.
Go to : 
In the last 2 decades, there have been numerous studies aimed at determining the gene expression signatures of tumors in order to better identify patients who could benefit from adjuvant systemic chemotherapy and those who could safely forgo it [1234]. The increased use of gene expression profiling has led to the omission of chemotherapy for patients with certain types of breast cancer.
Women younger than 35 years comprise 9.5%–12% of patients with breast cancer in Asia; this rate is higher than that of 4% found in Western countries [5]. Moreover, younger premenopausal women exhibit worse overall survival than older pre- and perimenopausal counterparts regardless of lymph node status or tumor size [6]. It has been proposed that the endocrine-associated effects of chemotherapy alone are insufficient to treat young women with estrogen receptor-positive breast cancer [78]. However, there is limited evidence that young patients with breast cancer gain a survival benefit from adjuvant tamoxifen after chemotherapy because of endocrine therapy resistance, which in turn necessitates additional treatments. Given that chemotherapy is thought to elicit ovarian function suppression (OFS) rather than producing a direct cytotoxic effect, such additional interventions include direct OFS using gonadotropin-releasing hormone (GnRH) agonists or surgical ablation. Premenopausal women usually experience improved survival after chemotherapy, and adding OFS may further reduce recurrence [9].
According to the Trial Assigning Individualized Options for Treatment (TAILORx), adjuvant endocrine and chemo-endocrine therapies had similar effects in women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, axillary node-negative breast cancer who had midrange 21-gene recurrence scores [1011]. The ‘Rx for Positive Node, Endocrine Responsive Breast Cancer’ study expanded the eligibility criteria of the TAILORx trial to include patients with 1–3 involved lymph nodes (as such patients are often considered to be at higher risk), and found that adjuvant chemotherapy does not benefit such patients who have a recurrence score of 25 or lower [1213]. Given this background, additional research may improve the identification of patients who can safely omit chemotherapy while accumulating additional evidence that OFS can in fact be a substitute for it.
To achieve this goal, it is necessary to compare the outcomes and genetic profiles of premenopausal patients who received only hormone therapy such as a GnRH agonist and tamoxifen to those who received chemotherapy combined with hormone therapy. Such studies may be of relevance given that the proportion of premenopausal patients is higher in Korea than in Western countries.
In this study, we compared survival rates between premenopausal patients with HR-positive breast cancer who underwent chemotherapy before OFS versus those who were only treated with a GnRH agonist and tamoxifen. Given that genetic profiles are currently insufficient indicators of treatment strategy, our findings ought to serve as a strong foundation for future prospective studies of this topic.
Go to : 
This study was approved by the Institutional Review Board of Korea Cancer Center Hospital (No. KIRAMS 2021-10-003), and the informed consent requirement was waived owing to its retrospective nature.
We retrospectively investigated the medical records of 5,741 patients who were newly diagnosed with primary invasive HR-positive, HER2-negative breast cancer between January 2009 and December 2019 and who underwent curative surgery. Only patients treated with a GnRH agonist were included, whereas the exclusion criteria included those with a history of other primary malignancies, de-novo stage IV breast cancer, pN2 breast cancer, pT3 breast cancer, and treatment with neoadjuvant chemotherapy or endocrine therapy.
Clinical parameters were compared using the chi-square test or Student t-test as appropriate. Disease-free survival was defined as the interval between diagnosis and the detection of progression; i.e., locoregional recurrence, distant metastasis, contralateral breast cancer, another primary tumor, or death from any cause. Invasive disease-free survival was defined as the interval between diagnosis and the detection of distant metastasis. Overall survival was defined as the interval between diagnosis and death from any cause. Survival analysis was performed using the Kaplan-Meier method, with differences assessed using the log-rank test.
For real-world comparisons, we used a propensity score matching (PSM) model by matching potentially confounding factors. Matching factors were age at diagnosis, pathologic T stage, pathologic N stage, histologic grade, and type of surgery. The match ratio for the 2 groups was 1:1 with a match tolerance of 0.1. Pathologic T and N stage and histologic grade were included as covariates when performing multivariate logistic regression for calculating the propensity score. A P-value of <0.05 was considered statistically significant.
Go to : 
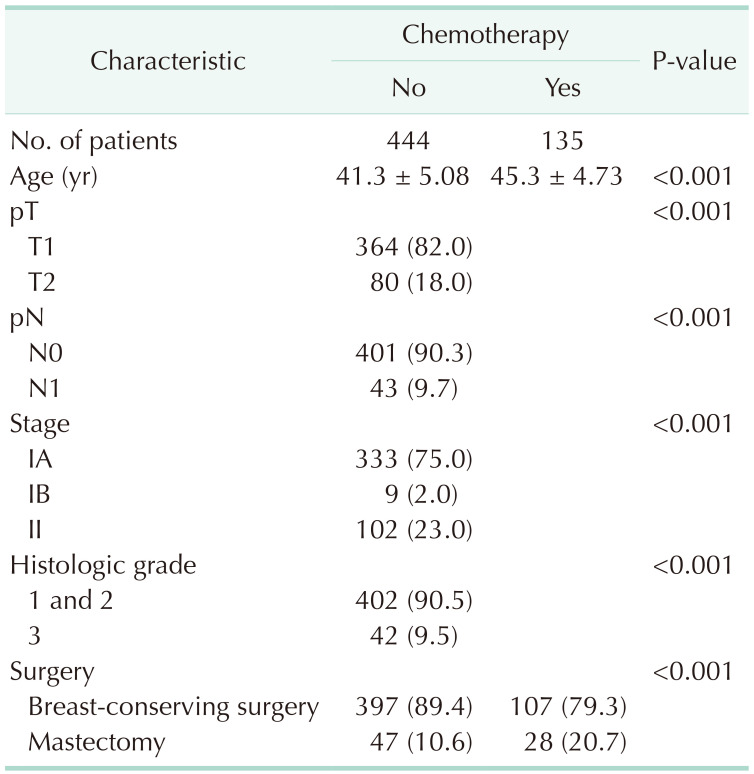
The patients’ demographics and characteristics of their tumors are presented in Table 1. Data from 579 patients were eligible for this analysis. Before PSM, the 444 patients in the non-chemotherapy group were older than the 135 in the chemotherapy group (45.3 ± 4.7 vs. 41.3 ± 5.1, P < 0.001). Patients in the non-chemotherapy group had significantly lower T and N stages than those in the chemotherapy group (P < 0.001); histological grades were also significantly different. There were more patients with N0 stage in the non-chemotherapy group than there were in the chemotherapy group (90.3% vs. 54.8%) while the opposite was true for patients with N1 stage (9.7% vs. 45.2%). The non-chemotherapy group comprised a greater proportion of patients with stage IA disease than did the chemotherapy group (75.0% vs. 31.9%) whereas the opposite was true for stage II disease (23.0% vs. 63.7%, P < 0.001). The non-chemotherapy group also comprised a greater proportion of patients with grades 1 and 2 disease than did the chemotherapy group (90.5% vs. 77.0%) whereas the reverse was true for grade 3 disease (9.5% vs. 23.0%, P < 0.001).
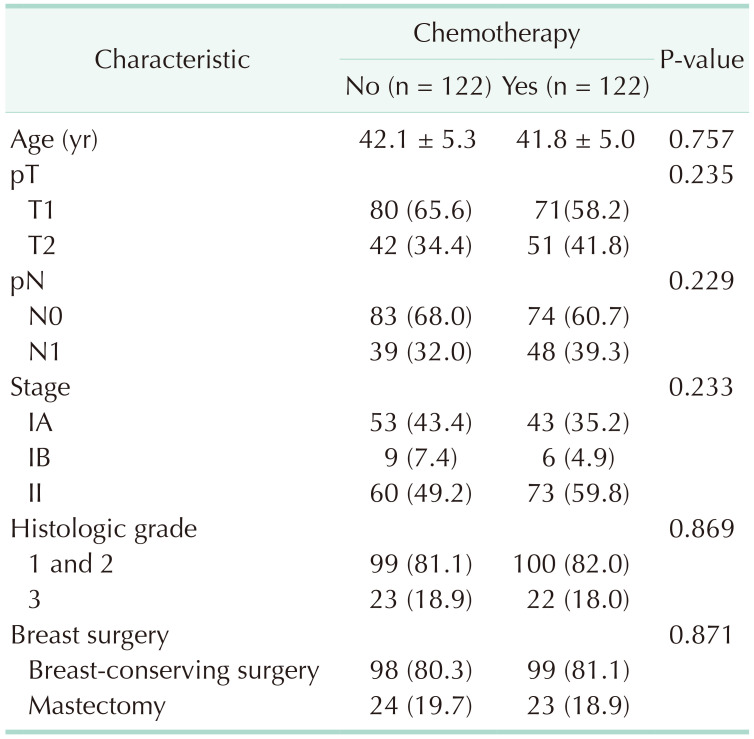
After PSM, 244 patients (122 in each group) were analyzed (Table 2). In contrast to findings before PSM, there were no significant differences in age at diagnosis, pathologic T and N stages or in histologic grade between the groups. The median follow-up period was 62.8 months (range, 2–144 months).
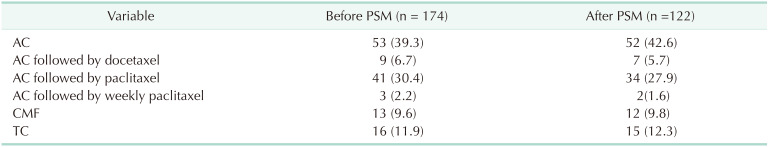
Several different chemotherapy regimens were used. Doxorubicin plus cyclophosphamide (AC), AC followed by docetaxel, AC followed by paclitaxel, AC followed by weekly paclitaxel, cyclophosphamide plus methotrexate and 5-fluorouracil, and docetaxel plus cyclophosphamide were used in the chemotherapy group (Table 3).
All patients received OFS. Goserelin was used for GnRH agonist and GnRH agonist was administered for 2 years. After diagnosis of breast cancer, 8 patients received oophorectomy and 3 patients received oophorectomy after the use of GnRH agonist. After 2 years of GnRH agonist administration, 4 patients were confirmed to have menopause and treatment was changed to aromatase inhibitor from tamoxifen. Aromatase inhibitors were used in 11 patients; 4 patients used letrozole and 7 patients used anastrozole.
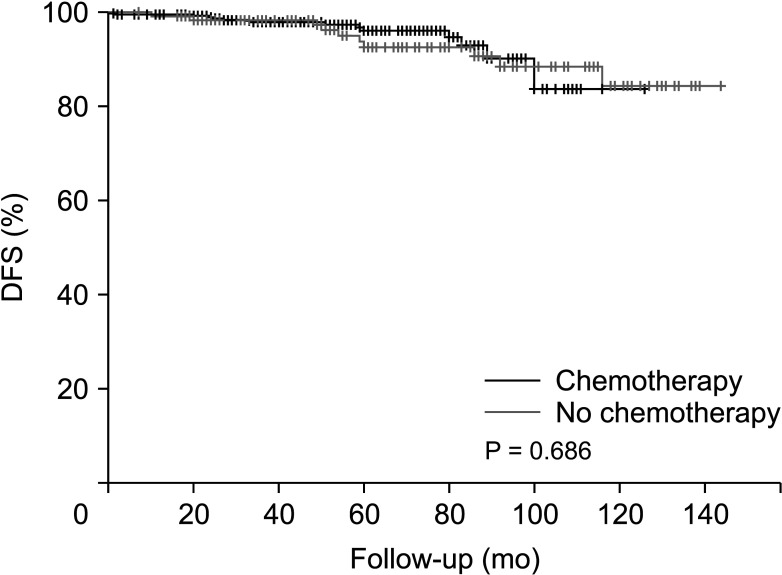
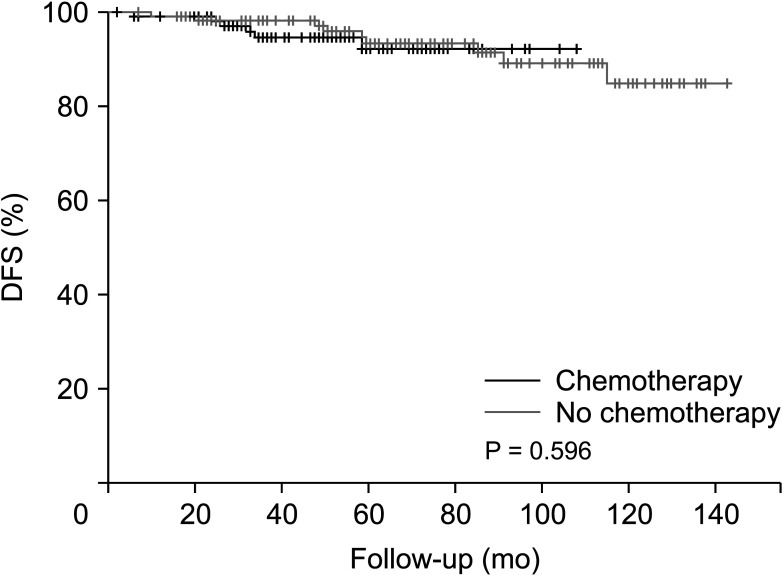
Before PSM, the disease-free survival was 128.7 months in the non-chemotherapy group and 137.4 months in the chemotherapy group (P = 0.686) (Fig. 1). After PSM, the disease-free survival was 118.1 months in the non-chemotherapy group and 133.8 months in the chemotherapy group (Fig. 2), with no significant difference between the groups (P = 0.596).
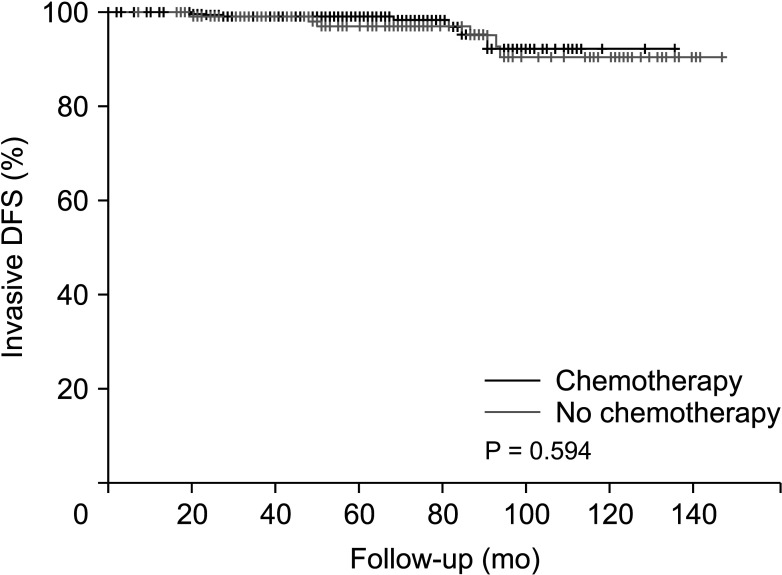
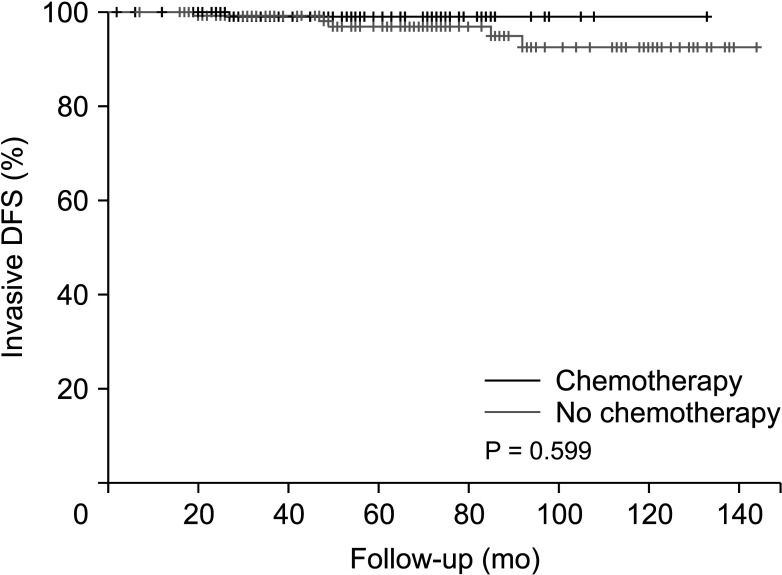
Invasive disease-free survival was 128.7 months in the non-chemotherapy group and 137.4 months in the chemotherapy group before PSM (P = 0.594) (Fig. 3). After PSM, invasive disease-free survival was 130.8 months in the non-chemotherapy group and 138.2 months in the chemotherapy group (P = 0.599) (Fig. 4).
Overall survival could not be calculated because all cases were censored; only a single patient (from the chemotherapy group) died.
Go to : 
We found that the outcomes of patients with HR-positive, HER2-negative N0 or N1 breast cancer who received only adjuvant endocrine therapy with OFS without chemotherapy were not inferior to those who received the same treatment with additional adjuvant chemotherapy. To the best of our knowledge, ours is the first study to demonstrate that adding chemotherapy to ovarian suppression and tamoxifen treatment does not provide an additional survival benefit for patients with HR-positive, HER2-negative breast cancer with pN0 and pN1 stages.
Nowadays, OFS alone or after chemotherapy or endocrine therapy alone is the main treatment option for patients with HR-positive, HER2-negative early-stage breast cancer [14]; however, the effectiveness of chemotherapy is unclear. Results from the TAILORx trial indicated that chemotherapy was beneficial in part owing to the inclusion of OFS among women aged <40, 40–45, and 45–50 years [11]. This might be explained (at least partially) by an ovarian suppression effect associated with premature menopause induced by chemotherapy; however, it remains unclear whether similar benefits could be achieved with OFS plus an aromatase inhibitor instead of chemotherapy [1516].
Sa-Nguanraksa et al. [17] demonstrated that premenopausal women with HR-positive, node-negative early breast cancer who received adjuvant treatment with GnRH agonist and tamoxifen had similar survival outcomes as those treated with adriamycin, cyclophosphamide, and tamoxifen, while their quality of life was actually improved. It was therefore suggested that the effect of chemotherapy in premenopausal women was in its eliciting OFS. Studies performed to date have compared tamoxifen plus chemotherapy or OFS plus chemotherapy to chemotherapy alone, given that these are standard treatments for premenopausal HR-positive, node-negative patients. However, there have been no studies comparing patients who underwent OFS without chemotherapy to those who received both treatments; even patients in all cohorts of the ‘Suppression of Ovarian Function Trial’ had undergone chemotherapy [18]. Our study is therefore meaningful given our evidence that OFS can substitute for chemotherapy.
Many chemotherapy agents have favorable toxicity profiles; however, a substantial number of patients might still experience toxicity owing to adjuvant chemotherapy without achieving clear benefits. Even though longer overall survival is well-established, the adverse effects of chemotherapy (whether acute or late occurring) are a major factor in treatment outcomes and quality of life. Moreover, remedies administered to relieve such adverse effects have their own setbacks; for example, the risk of developing secondary leukemia doubles following the administration of granulocyte colony-stimulating factor [192021]. There is also an increased risk of leukemia and myelodysplastic syndromes in patients with breast cancer who have been treated with alkylating agents and topoisomerase-II inhibitors such as anthracyclines [22]. Moreover, alopecia is considered one of the most notable side effects of chemotherapy for patients with breast cancer and can cause them to refuse treatment owing to the distress and trauma of hair loss even if clinicians do not consider this a serious complication [23].
Limitations of our study included that it was retrospective and was performed at a single institution. Moreover, both groups included premenopausal women only with a mean age under 50 years. Considering the side effects and uncertain effectiveness of chemotherapy, it will be important to identify patients in whom such treatment can safely be omitted, and our study ought to serve as a starting point for wider-scope randomized trials that involve testing gene expression.
In conclusion, we demonstrated the lack of any additional benefit to adding chemotherapy to OFS treatment of premenopausal HR-positive HER2-negative patients with pN0 and pN1 breast cancer patient. These results could be the basis for larger-scale prospective studies.
Go to : 
References
1. Salgado R, Peg V, Rüschoff J, Vincent-Salomon A, Castellano I, Perner S, et al. Gene expression signatures for tailoring adjuvant chemotherapy of luminal breast cancer: the pathologists’ perspective. Ann Oncol. 2021; 32:1316–1321. PMID: 34461263.
2. Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009; 360:790–800. PMID: 19228622.
3. Azim HA Jr, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M, et al. Utility of prognostic genomic tests in breast cancer practice: the IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013; 24:647–654. PMID: 23337633.
4. Vieira AF, Schmitt F. An update on breast cancer multigene prognostic tests-emergent clinical biomarkers. Front Med (Lausanne). 2018; 5:248. PMID: 30234119.
5. Winchester DP. Breast cancer in young women. Surg Clin North Am. 1996; 76:279–287. PMID: 8610264.
6. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea. A report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368. PMID: 17515570.
7. Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thürlimann B, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000; 355:1869–1874. PMID: 10866443.
8. Kangleon-Tan HL, Sim J, You JY, Lee ES, Lee H, Yang SM, et al. Omission of chemotherapy for hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: patterns of treatment and outcomes from the Korean Breast Cancer Society Registry. Ann Surg Treat Res. 2022; 103:313–322. PMID: 36601341.
9. Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010; 362:2053–2065. PMID: 20519679.
10. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018; 379:111–121. PMID: 29860917.
11. Sparano JA, Gray RJ, Ravdin PM, Makower DF, Pritchard KI, Albain KS, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019; 380:2395–2405. PMID: 31157962.
12. Gonzalez-Angulo AM, Barlow WE, Gralow J, Meric-Bernstam F, Hayes DF, Moinpour C, et al. SWOG S1007: A phase III, randomized clinical trial of standard adjuvant endocrine therapy with or without chemotherapy in patients with one to three positive nodes, hormone receptor (HR)-positive, and HER2-negative breast cancer with recurrence score (RS) of 25 or less. J Clin Oncol. 2011; 29(15 Suppl):TPS104.
13. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.
14. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020; 18:452–478. PMID: 32259783.
15. Chung CC, Fleming R, Jamieson ME, Yates RW, Coutts JR. Randomized comparison of ovulation induction with and without intrauterine insemination in the treatment of unexplained infertility. Hum Reprod. 1995; 10:3139–3141. PMID: 8822431.
16. Montemurro F, Perrone F, Geuna E. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015; 372:1672–1673.
17. Sa-Nguanraksa D, Krisorakun T, Pongthong W, O-Charoenrat P. Survival outcome of combined GnRH agonist and tamoxifen is comparable to that of sequential adriamycin and cyclophosphamide chemotherapy plus tamoxifen in premenopausal patients with early breast cancer. Mol Clin Oncol. 2019; 11:517–522. PMID: 31620283.
18. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018; 379:122–137. PMID: 29863451.
19. Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy. Anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018; 9:245. PMID: 29623040.
20. Azim HA Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011; 22:1939–1947. PMID: 21289366.
21. Al-Mahayri ZN, Patrinos GP, Ali BR. Toxicity and pharmacogenomic biomarkers in breast cancer chemotherapy. Front Pharmacol. 2020; 11:445. PMID: 32351390.
22. Park MJ, Park YH, Ahn HJ, Choi W, Paik KH, Kim JM, et al. Secondary hematological malignancies after breast cancer chemotherapy. Leuk Lymphoma. 2005; 46:1183–1188. PMID: 16085560.
23. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008; 17:317–328. PMID: 17721909.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download