Abstract
Purpose
Although the overall survival (OS) of breast cancer patients is increasing with improved detection and therapies, so is the risk of breast cancer patients developing subsequent malignancies. We investigated the OS of breast cancer survivors according to sites of second primary malignancies (SPM). The OS of the second primary hematologic malignancy (SPHM) was then compared with that of metastatic breast cancer (MBC).
Methods
We retrospectively analyzed patients diagnosed with primary breast cancer between 1998 and 2019. Only those with SPM were eligible for analysis. First, the OS of patients with SPM diagnosed as the first event after the diagnosis of breast cancer was analyzed. Next, the OS of patients with SPHM, with or without breast cancer relapse, was compared with that of patients with MBC, matched using the propensity score.
Results
Patients diagnosed with SPM without breast cancer relapse as the first event had a significantly better OS than did patients with MBC, but the OS of those with SPHM as the first event did not differ significantly from that of patients with MBC (hazard ratio [HR], 1.558; 95% confidence interval [CI], 0.856–2.839; P = 0.147). The OS of patients with SPHM with or without breast cancer relapse was worse than that of the MBC group after propensity score matching (HR, 1.954; 95% CI, 1.045–3.654; P = 0.036).
Breast cancer is the most common cancer in women worldwide. The overall survival (OS) of breast cancer patients has improved dramatically with advancements in treatment strategies and improvement in socioeconomic support [12]. Consequently, the incidence of patients diagnosed with a second primary malignancy (SPM) after primary breast cancer has increased [3]. SPMs have been studied among Korean breast cancer survivors [4]. The long-term complications of anticancer therapy are becoming more prominent with prolonged survival of patients.
The development of SPM after primary breast cancer may be affected by multiple factors, including the late toxicity of anticancer therapy. The relationship between the development of endometrial cancer and the use of tamoxifen has been investigated, and taking a longer duration of tamoxifen was revealed as a cause of endometrial cancer [5]. The cytotoxic chemotherapy and radiotherapy used to treat primary breast cancer may be significant in the development of SPMs, such as hematologic malignancy or lung cancer [2]. Specifically, certain alkylating agents and platinum compounds, and supportive treatment with granulocyte colony-stimulating factor are associated with SPM development. However, the incidence of these treatment-related malignancies is relatively low.
Although SPM is very rare compared to breast cancer recurrence, its diagnosis is a terrifying experience for breast cancer survivors. In most cases, the physician faces the following question from the patient: “Is the prognosis of my second primary cancer better than that of breast cancer metastasis, or not?” Because of the scarcity of reported data on the survival after SPMs and finely subdivided modern specialties of physicians, discussions regarding the prognosis of SPM from various organs are usually based on assumptions rather than evidence. In particular, discussion with a patient diagnosed with second primary hematologic malignancy (SPHM) could be difficult because of the poor prognosis of hematologic malignancy as primary cancer and the lack of reliable data on the prognosis of SPHM in breast cancer patients. To address this gap in knowledge, we conducted a retrospective analysis using propensity score matching to provide insights that could be helpful for both physicians and patients in the clinic, especially focusing on SPHMs: a possible treatment-related cancer.
In this study, we analyzed the survival of patients with SPM, which was diagnosed as the first event during the follow-up period after active treatment for early breast cancer. In addition, the OS of patients with SPHMs, with or without breast cancer relapse, was compared with that of patients with breast cancer distant metastasis using propensity score matching analysis.
This retrospective study was conducted using a chart review. The study was approved by the Institutional Review Board of the Korea Cancer Center Hospital (No. 2021-11-003). The need for informed consent was waived because all data were collected retrospectively and de-identified.
Patients diagnosed with primary breast cancer at our institute between 1998 and 2019 were screened for eligibility. The inclusion criteria were as follows: histological diagnosis of primary breast cancer as invasive ductal carcinoma, invasive lobular carcinoma, or ductal carcinoma in situ. The exclusion criteria were as follows: malignant phyllodes tumor, breast lymphoma, breast sarcoma, lobular carcinoma in situ, history of malignancy of another organ before the diagnosis of primary breast cancer, or synchronous cancer of another organ diagnosed with primary breast cancer. Among the patients, we chose those with metastatic breast cancer without SPM (group A, n = 995). We then selected all patients diagnosed with SPM as the first event after breast cancer diagnosis and did not have breast cancer metastasis (group B, n = 219). For specific analysis with SPHM, we selected all patients who were diagnosed with SPHM as the first event after active treatment for early breast cancer without evidence of breast cancer recurrence and those who were diagnosed with SPHM having de novo stage IV breast cancer or breast cancer relapse (group C). The medical records of the patients were reviewed to collect data on clinic-pathologic characteristics, treatments received, and survival outcomes.
SPM was defined as malignancy of another organ diagnosed as the first event after the diagnosis of primary breast cancer. SPHM was defined as any type of leukemia, lymphoma, multiple myeloma, and myelodysplastic syndrome that was diagnosed after diagnosing primary breast cancer. Distant metastasis was defined as breast cancer that had spread from the breast to distant organs or distant lymph nodes, as confirmed by radiologic or pathologic examination, regardless of whether it was found at the time of primary breast cancer diagnosis or developed as the first event during the follow-up period after curative surgery for primary breast cancer.
Categorical variables and continuous variables with 2 groups were compared using the chi-square test or Student t-test, respectively. Kaplan-Meier curves were utilized to determine the OS, and differences were assessed using the log-rank test. OS was defined as the time interval from the date of diagnosis of SPM, SPHM, or distant metastasis of breast cancer to the date of death by any cause. Patients who were alive at the last follow-up were censored.
Two sets of analyses were conducted (Fig. 1). First, we compared the OS of group A patients (n = 995) diagnosed with breast cancer metastasis as the first event during or after breast cancer treatment with the OS of group B patients (n = 219) diagnosed with SPM without breast cancer metastasis (set 1 analysis). Group B was classified according to types of cancer and OS of each cancer type was compared with group A having metastatic breast cancer without SPM. Next, we used propensity score matching analysis to focus on the SPHM (set 2 analysis). To balance the differences in prognostic variables associated with OS after the diagnosis of SPHM or breast cancer metastasis, we generated 1:2 SPHM (group C) and breast cancer metastasis-matched cohorts (group A') by propensity score matching. The OS of group C (n = 42) was analyzed and compared with those of group A' (n = 84). The propensity score was estimated based on R software ver. 4.3 (R Foundation for Statistical Computing) with the following covariates: age at diagnosis of primary breast cancer, age at diagnosis of SPHM or breast cancer distant metastasis, M stage of primary breast cancer, and breast cancer subtypes classified based on the expression of hormone receptor and human epidermal growth factor receptor 2 (HER2) status. Statistical significance was set at P < 0.05.
In total, 10,153 patients with primary breast cancer diagnosed between 1998 and 2019 were screened for eligibility. Among those eligible patients, 995 people were classified as group A: metastatic breast cancer patients but not having SPM. Two hundred nineteen patients were diagnosed with SPM as the first event without breast cancer recurrence and they were classified as group B. Forty-two patients who were diagnosed with SPHM as the first event regardless of breast cancer relapse were defined as group C (Fig. 1). The median follow-up period was 69 months (range, 1–277 months).
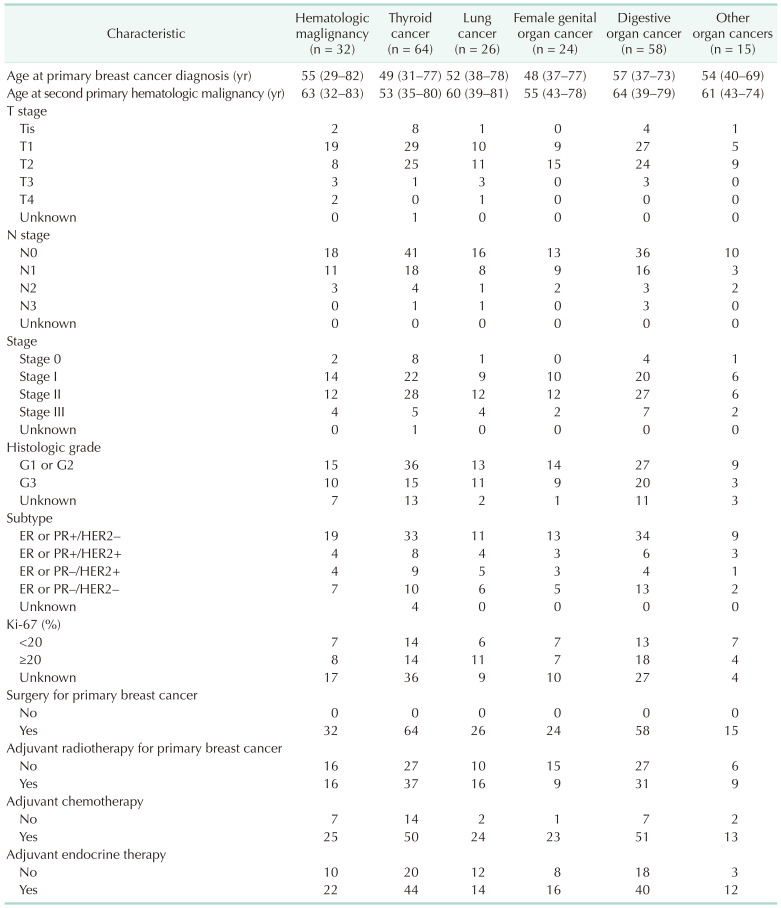
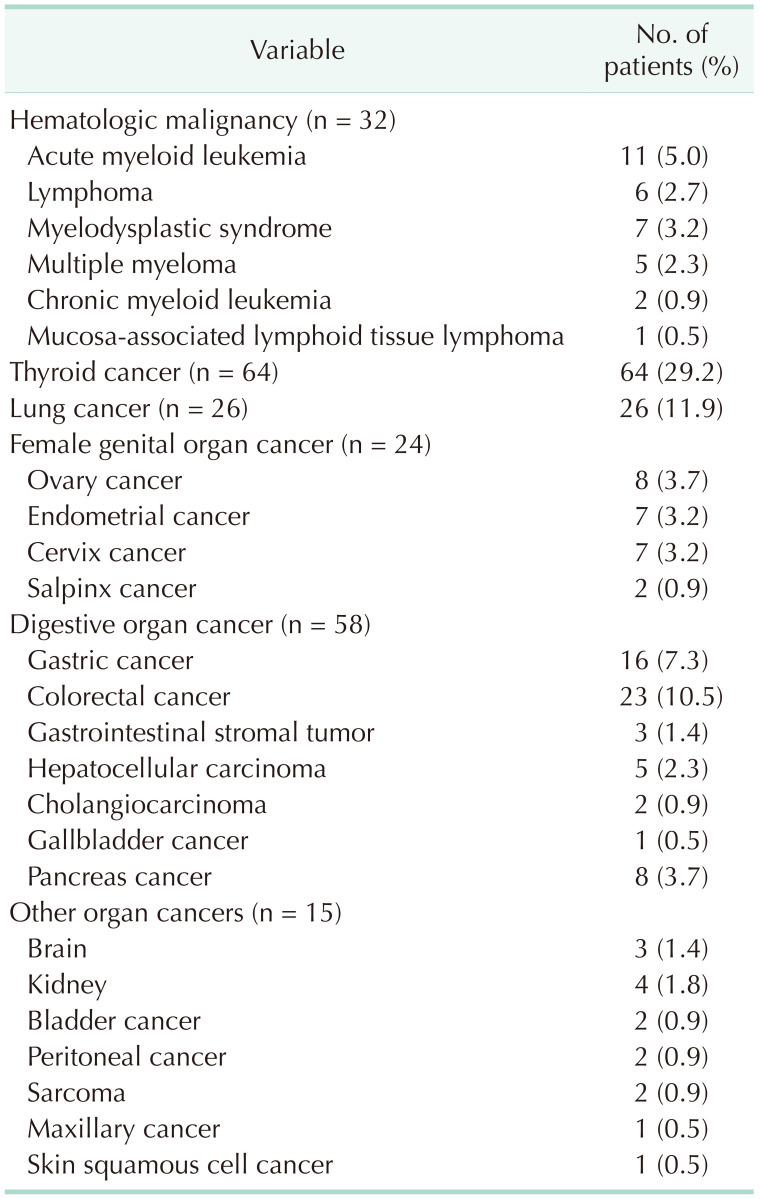
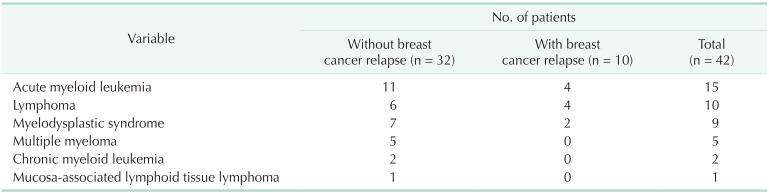
We classified group B patients according to the type of secondary primary malignancy. Table 1 shows the number of breast cancer patients who were diagnosed with SPM as a first event. Thyroid cancer was the most common SPM diagnosed as the first event (64 patients, 29.2%). Lung cancer was diagnosed in 26 patients (11.9%), colorectal cancer in 23 patients (10.5%), and gastric cancer in 16 patients (7.3%). Of group B patients, SPHM was diagnosed as the first event during the follow-up period after curative surgery for breast cancer in 32 patients. Acute myeloid leukemia (AML) was the most common SPHM, followed by myelodysplastic syndrome. One of the patients diagnosed with myelodysplastic syndrome as the first event developed AML during treatment for myelodysplastic syndrome and died due to AML. Clinicopathologic characteristics of breast cancer patients with SPM are summarized in Table 2.
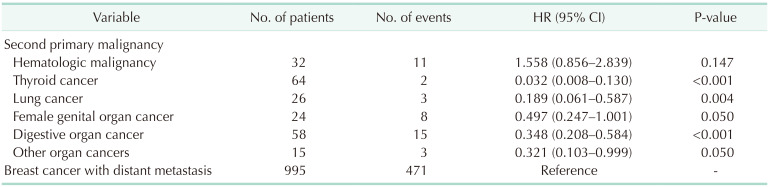
Patients with SPM, except those with SPHM, showed better OS than metastatic breast cancer patients without SPM (Fig. 2). The OS of patients with SPHM diagnosed as the first event was not significantly different from that of patients with breast cancer metastasis (hazard ratio [HR], 1.558; 95% confidence interval [CI], 0.856–2.839; P = 0.147) (Table 3).
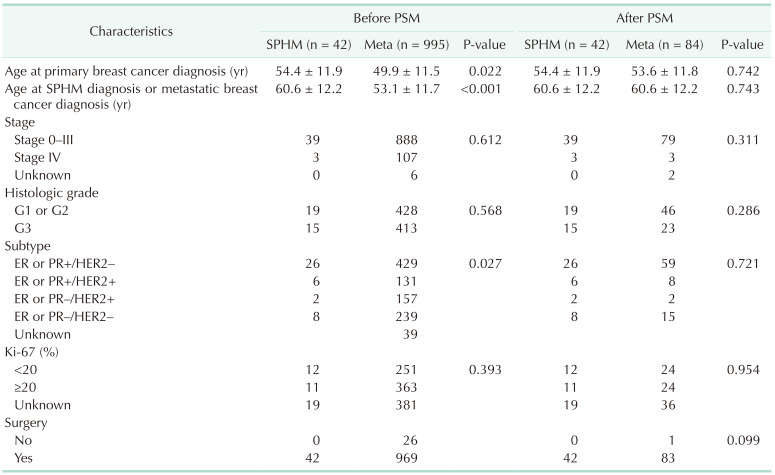
Including 32 cases of SPHM as the first event during the follow-up period (SPHM subgroup of group B), a total of 42 patients were analyzed in set 2 analysis (Fig. 1, Table 4). Ten patients were identified with SPHM after the diagnosis of de novo stage IV breast cancer or any type of breast cancer relapse. Of these patients, SPHM was found in 2 patients after the diagnosis of contralateral breast cancer, 5 after metastatic breast cancer recurrence, and 3 during treatment for stage IV breast cancer. After propensity score matching with patients with metastatic breast cancer without SPM (group A), there were 42 and 84 patients in the SPHM and metastatic breast cancer groups, respectively.
Before adjustment by propensity score matching, there was a statistically significant difference between the groups in age at the time of diagnosis of primary breast cancer, age at SPHM diagnosis or breast cancer distant metastasis, and breast cancer subtypes classified based on the expression of prognostic hormone receptor and HER2. After propensity score matching, these clinicopathologic variables were balanced (Table 5).
Before propensity score matching analysis, the OS of the SPHM group was worse than that of the metastatic breast cancer group (HR, 1.842; 95% CI, 1.116–3.039; P = 0.017) (Fig. 3A). The OS after PSM remained worse in the SPHM group than that in the metastatic breast cancer group (HR, 1.954; 95% CI, 1.045–3.654; P = 0.036) (Fig. 3B).
In this study, patients with SPHM showed worse OS than did patients with breast cancer metastasis, both before and after propensity score matching. However, the OS of patients with other SPMs was better than that of patients with metastatic breast cancer. To the best of our knowledge, this is the first report comparing the OS of breast cancer patients with SPHM with that of those with metastatic breast cancer.
According to the National Cancer Statistics in Korea 2019 report by the Korea Central Cancer Registry, which was initiated by the Ministry of Health and Welfare of the Republic of Korea, breast cancer is the most common malignancy in women in the Republic of Korea, followed by thyroid cancer, colorectal cancer, gastric cancer, and lung cancer [6]. In this study, the most common SPMs that were diagnosed as the first event after primary breast cancer surgery were thyroid cancer, hematologic malignancy, lung cancer, colorectal cancer, and gastric cancer. Except for hematologic malignancies, the results were concordant with the report of the National Cancer Statistics in Korea. Therefore, we assumed that the incidence of SPMs in this study may be reliable, although it was investigated only in a single institute. Hematologic malignancy is notorious as treatment-related cancer in breast cancer survivors [78]. A nationwide cohort study conducted in France showed that the incidence of hematologic malignant neoplasms was significantly higher among breast cancer survivors than among women in the general population for AML, myelodysplastic syndrome, multiple myeloma, acute lymphocytic leukemia, and lymphocytic lymphoma, after age standardization [9]. An analysis of the SEER (Surveillance, Epidemiology, and End Results) program showed that the risk of treatment-related myelodysplastic syndrome and AML was significantly elevated after the diagnosis of breast cancer [10]. Taken together, the relatively higher incidence of hematologic malignancy in breast cancer patients in this study than the prevalence of hematologic malignancy in the general population of the Republic of Korea might be associated with the treatment of previous breast cancer.
In addition, patients with SPHM showed relatively short follow-up periods of less than 60 months, even compared with patients with SPM. Regardless of whether it is treatment-related, aggressive features and poor survival of hematologic malignancy could be the reason for the difference in the survival curve in Fig. 2. However, we cannot predicate that prognosis of hematologic malignancy is always worse than solid tumors. Survival outcome depends on the type of cancer, staging, and individual patient factors. Therefore, we added Supplementary Table 1 to provide information on the TNM staging of SPM. Some patients with SPM were diagnosed with stage IV, but most were stage I–III. Considering that stage is associated with prognostic factors, it could be anticipated that group B showed better survival than group A. Therefore, for patients with breast cancer, identifying whether the newly found lesion is metastasis from breast cancer or other primary cancer is crucial.
In our practice, patients diagnosed with SPHM were referred to hematologic specialists and received appropriate treatment. Similarly, patients with SPM were treated by a qualified surgical or medical oncologist for each organ.
This study was initiated in response to the frequent question of whether or not the prognosis of SPM was better than that of breast cancer metastasis in patients diagnosed with SPM after a primary diagnosis of breast cancer. The answer may be complicated by the heterogeneity of breast cancer. The prognosis of metastatic breast cancer could vary according to clinicopathologic characteristics, such as the hormone receptor status, HER2 overexpression, age of patients, menopausal status, or time interval from breast cancer diagnosis to relapse [11]. However, the subtypes of primary breast cancer are not associated with either SPM or SPHM (Table 2). The prognosis of patients with SPMs also varies according to the organ of origin. In addition, recognition of their general prognosis is difficult because of the relatively small number of patients with SPMs and the consultation provided by physicians from different departments, according to the type of SPM in the modern era. In this analysis, we categorized SPMs according to the organs in which the malignancy originated to evaluate the difference in OS between SPMs and compare them with the OS of patients with metastatic breast cancer. As a result, except for SPHM, all groups of SPMs showed better OS than the group with metastatic breast cancer. Therefore, we focused on the comparison of OS between SPHM and metastatic breast cancer and conducted a propensity score matching analysis to minimize bias. However, even after propensity score matching analysis, the OS of patients with SPHM was worse than that of patients with metastatic breast cancer.
The reported OS following SPHM after the diagnosis of various solid tumors was poor [9]. In a review of the SEER database, OS following therapy-related myelodysplastic syndrome or AML was poor, and 1,270 of 1,619 patients (78.4%) died. The median OS was 7 months [10]. The risk of developing SPHM was influenced by chemotherapy, particularly alkylating agents and topoisomerase inhibitors [12]. As these chemotherapeutic agents play a key role in the treatment of early breast cancer, the incidence of treatment-related SPHM has consequently increased [3]. Fortunately, gene expression assays, such as Oncotype Dx (Exact Sciences), MammaPrint (Agendia), Prosigna (NanoString Technologies), and EndoPredict (Myriad Genetics) have been introduced and are actively used to guide treatment decisions in patients with hormone-sensitive breast cancer [781314]. As a result, physicians and patients have a reliable tool to avoid the risk of over-treatment by using chemotherapy in selected patients, and the era of deescalated treatment has opened up. It could also reduce the risk of treatment-related SPHM and the long-term toxicity of chemotherapy.
In conclusion, patients with SPHM showed worse survival than did patients with breast cancer metastasis, even after adjusting for clinicopathological values by propensity score matching. Except for SPHM, patients diagnosed with SPMs showed better survival than those with breast cancer metastasis. These results may help both physicians and patients while discussing secondary cancer in the clinic.
Notes
References
1. Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, et al. Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021; 24:123–137. PMID: 33913273.
2. Long Q, Wang Y, Che G. Primary lung cancer after treatment for breast cancer. Int J Womens Health. 2021; 13:1217–1225. PMID: 34908880.
3. Kaplan HG, Malmgren JA, Atwood MK. Increased incidence of myelodysplastic syndrome and acute myeloid leukemia following breast cancer treatment with radiation alone or combined with chemotherapy: a registry cohort analysis 1990-2005. BMC Cancer. 2011; 11:260. PMID: 21693006.
4. Jung HK, Park S, Kim NW, Lee JE, Kim Z, Han SW, et al. Development of second primary cancer in Korean breast cancer survivors. Ann Surg Treat Res. 2017; 93:287–292. PMID: 29250506.
5. Emons G, Mustea A, Tempfer C. Tamoxifen and endometrial cancer: a Janus-headed drug. Cancers (Basel). 2020; 12:2535. PMID: 32906618.
6. Ministry of Health and Welfare. Cancer Registration Statistics: number of cancer cases and incidence rates by 24 cancer types/sex/age (in 5-year intervals) [Internet]. Ministry of Health and Welfare;2023. cited 2023 Jun 26. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A00023&conn_path=I2.
7. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–3734. PMID: 16720680.
8. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.
9. Jabagi MJ, Vey N, Goncalves A, Le Tri T, Zureik M, Dray-Spira R. Evaluation of the incidence of hematologic malignant neoplasms among breast cancer survivors in France. JAMA Netw Open. 2019; 2:e187147. PMID: 30657534.
10. Morton LM, Dores GM, Schonfeld SJ, Linet MS, Sigel BS, Lam CJ, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019; 5:318–325. PMID: 30570657.
11. Colozza M, de Azambuja E, Personeni N, Lebrun F, Piccart MJ, Cardoso F. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007; 12:253–270. PMID: 17405890.
12. Kaplan HG, Calip GS, Malmgren JA. Maximizing breast cancer therapy with awareness of potential treatment-related blood disorders. Oncologist. 2020; 25:391–397. PMID: 32073195.
13. Piccart M, van't Veer LJ, Poncet C, Lopes Cardozo JM, Delaloge S, Pierga JY, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021; 22:476–488. PMID: 33721561.
14. Villarreal-Garza C, Ferrigno AS, De la Garza-Ramos C, Barragan-Carrillo R, Lambertini M, Azim HA Jr. Clinical utility of genomic signatures in young breast cancer patients: a systematic review. NPJ Breast Cancer. 2020; 6:46. PMID: 33062888.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2023.105.1.1.
Supplementary Table 1
TNM staging of patients with second primary solid malignancy as a first event
Fig. 1
Description of the study analysis. SPM, second primary malignancy; SPHM, second privary hematologic malignancy; MBC, metastatic breast cancer.
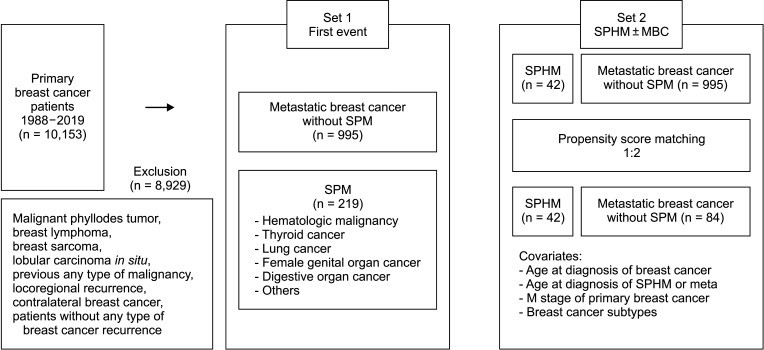
Fig. 2
Overall survival of metastatic breast cancer vs. the second primary malignancy diagnosed as the first event.
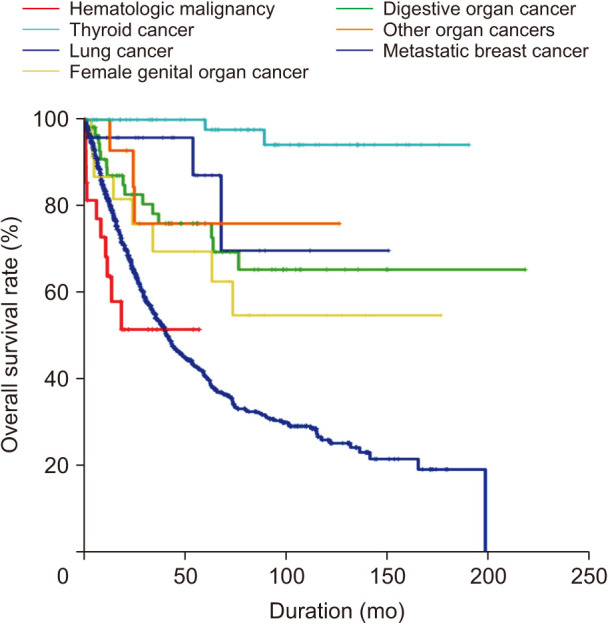
Fig. 3
Overall survival of all second primary hematologic malignancies and metastatic breast cancer before (A) and after (B) propensity score matching.
Table 2
Clinicopathologic characteristics of patients with second primary malignancy as a first event




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download