Abstract
Purpose
B-cell lymphoma 2 (BCL2) has an antiapoptotic role, however, has resulted in it being a powerful favorable prognostic factor in breast cancer. Several studies revealed BCL2 is strongly associated with a lower rate of early recurrence after initial treatment in breast cancer patients, but study of a prolonged effect after 5 years is lacking. We investigated BCL2 as a prognostic factor in breast cancer in comparison to early and late recurrence.
Methods
We retrieved data from 2,198 patients with primary breast cancer who underwent surgical treatment and adjuvant treatment at the breast cancer center between 2005 and 2015. Each molecular subtype was classified, and Ki-67 and BCL2 were also assessed by immunohistochemistry. BCL2 and the association between molecular subtypes were assessed in early and late recurrences, respectively. Five-year postrecurrence survival and BCL2 were also assessed.
Results
The BCL2-positive group was associated with favorable clinicopathologic characteristics. The time to recurrence was significantly longer in the BCL2-positive group (P = 0.035). Late recurrence after 5 years was higher in the BCL2-positive group (P = 0.029). In multivariate survival analysis, tumor size and BCL2-positive expression were the only independent prognostic factors for late recurrence (P = 0.004). In the patients with recurrence, 5-year postrecurrence survival was significantly higher in the BCL2-positive group (P < 0.001).
Conclusion
Our result showed that prognosis was better in BCL2-positive patients compared to BCL2-negative patients at late recurrence. We suggested that BCL2 expression could be used as a marker to help determine additional adjuvant therapy or extended hormone therapy in hormone-dependent breast cancer.
Go to : 
Breast cancer is a highly heterogeneous disease and includes different molecular subtypes. This heterogeneity makes it difficult to predict the prognosis of breast cancer patients and influences tumor development and progression [12].
The genetic features of the tumor and adjuvant therapy methods affect the risk and timing of recurrence [345]. In hormone receptor-negative breast cancer, recurrences mostly occur within the first 5 years after diagnosis, and the relapse rates drop rapidly thereafter, whereas late recurrences more than 5 years after diagnosis consistently occur in patients with hormone receptor-positive breast cancer [67].
A range of techniques, such as clinicopathological variables and multigene assays, are employed to evaluate the late recurrence of hormone receptor-positive breast cancer [8910]. It is needed to identify patients at risk of late recurrence of breast cancer and establish treatment strategies such as extended endocrine therapy. This is a way to mitigate the toxicity burden of extended endocrine therapy in breast cancer patients. The clinical treatment score at 5 years in breast cancer is a late recurrence predictor that includes tumor size, the number of positive nodes, histologic grade, and age and is used to estimate an individual’s risk of late, distant metastatic recurrence [11].
B-cell lymphoma 2 (BCL2) is a key player in the regulation of cell death and is recognized as an oncogene in a variety of malignancies, particularly leukemia and lymphoma [12]. BCL2 overexpression inhibits apoptotic cell death and activates cell proliferation and tumor progression [12]. Many studies have examined the clinical importance of BCL2 expression and concluded that BCL2 is an independent and powerful prognostic protein marker, in hormone receptor-positive breast cancer [13].
The aim of this study was to examine the relationship between late breast cancer recurrence and BCL2, which is easily accessible in clinical practice, and other known clinical pathological variables.
Go to : 
This study was approved by the Institutional Review Board of our hospital (No. KC 15RISI0804). The study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
A prospective cohort of patients with invasive breast cancer who underwent surgical treatment and adjuvant treatment was reviewed retrospectively at a single institute. A total of 3,130 patients were diagnosed with invasive breast cancer between January 2005 and December 2015.
We excluded patients with non-invasive carcinoma, distant metastasis at diagnosis, bilateral breast cancer, and any other malignancy. Patients who had insufficient clinicopathologic data or had received neoadjuvant treatment were excluded.
Patients’ clinical and pathological features including age at diagnosis, type of surgery, pathological T and N staging, breast cancer stage according to the 8th edition of American Joint Committee on Cancer (AJCC) classification, histologic grade, type of adjuvant treatment (chemotherapy, endocrine therapy, and radiotherapy), Ki-67 status, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2 (HER 2) expression, molecular subtype and incidence of recurrence and death were reviewed.
For ER (SP1, prediluted; Roche), PR (1E2, prediluted; Roche), HER2 (4B5, prediluted; Roche), Ki-67 (MIB-1, prediluted; Roche), and BCL2 (124, 1:50; Dako), immunohistochemistry (IHC) was performed on whole tissue section slides using BenchMark ULTRA (Ventana Medical Systems). A positive ER and PR status was defined as an Allred score of ≥3. HER2 was defined as negative when the IHC score was 0 or 1+, and defined as positive as an IHC score of 3+. If the IHC score was 2+, the sample was retested with fluorescence in situ hybridization. The Ki-67 positive cut-off level was level ≥14% according to the expressing cell ratio. BCL2 expression was determined by analyzing the percentage of positively stained cells. BCL2 expression was interpreted by a single pathologist on the basis of staining intensity and extent. BCL2-positive was defined when >10% of the cells were stained.
Four intrinsic molecular subtypes were derived based on slight modifications of methods described in the 13th St. Gallen International Expert Consensus: (1) luminal A: ER- and/or PR-positive, HER2-negative, and a low Ki-67 level (<14%); (2) luminal B: ER- and/or PR-positive, HER2-positive, and any Ki-67 level; (3) HER2-overexpression: HER2 overexpression or amplification, and absence of ER and PR expression; and (4) triple-negative breast cancer (TNBC): ER-, PR-, and HER2-negative [14]. All cancers were staged according to the eighth edition of the AJCC staging manual.
Based on 5 years, it was divided into early and late recurrences. Early recurrence was defined as the occurrence of any breast cancer-related event within 5 years of the first diagnosis of breast cancer, including local, regional, and distant recurrences and death from breast cancer. Late recurrence was defined as any breast cancer-related event that occurred 5 years after the first diagnosis of breast cancer. Any non-breast cancerrelated death was censored. Postrecurrence overall survival (P-OS) was calculated from the time of diagnosis of the first recurrence to the time of death from breast cancer.
The chi-square and Fisher exact tests were used for comparison between categorical variables. Cumulative survival probabilities were estimated using the Kaplan-Meier analysis. The log-rank test was performed for comparing differences between survival rates. A multivariate analysis was performed using the Cox proportional hazards regression model. All tests were 2-sided and a P-value of <0.05 was considered to be statistically significant. The statistical analyses were performed with I PASW Statistics software ver. 18.0 for Windows (IBM Corp.).
Go to : 
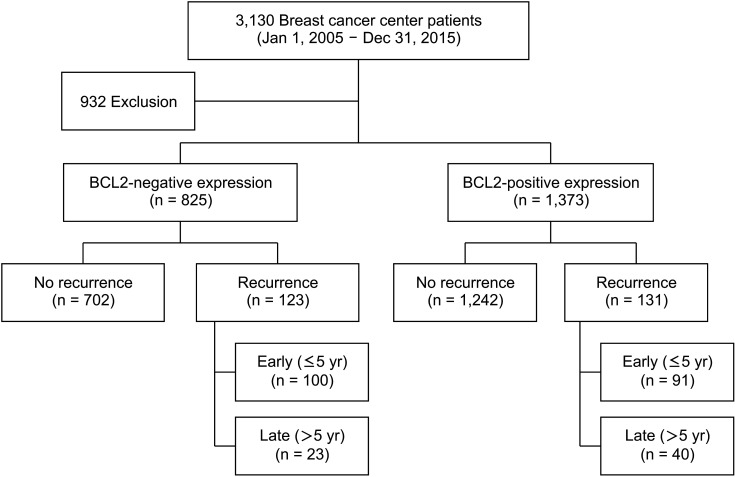
From 2005 to 2015, a total of 3,130 patients were operated on for breast cancer. Excluding patients who meet the exclusion criteria, 2,198 were included in the statistical analysis. There were 254 recurrences (11.6%), 191 recurrences occurred within 5 years, and 63 recurrences after 5 years (Fig. 1).
The mean patient age was 51.59 ± 0.23 years, and the mean follow-up was 171.18 ± 0.79 months. The clinical and pathological characteristics of the 2,198 patients are summarized in Table 1. BCL2-positive expression was observed in 1,373 patients (62.5%), and BCL2-negative expression was observed in 825 patients (37.5%). The BCL2-positive group was significantly different from the BCL2-negative group in terms of age, axillary operation, tumor size, nodal status, pathologic stage, histologic grade, ER status, PR status, HER2 status, Ki-67, molecular subtype, adjuvant chemotherapy, adjuvant endocrine therapy, and incidence of recurrence and death. The BCL2-positive group was significantly associated with younger age (P = 0.021), smaller tumor size (P = 0.001), lower rate of lymph node (LN) metastasis (P < 0.001), early-stage disease (P < 0.001), lower histologic grade (P < 0.001), hormone receptor positivity (P < 0.001), HER2 negativity (P < 0.001), lower Ki-67 (≥14%) (P < 0.001), higher rate of luminal molecular subtype (P < 0.001), and higher rate of adjuvant chemotherapy or endocrine therapy (P < 0.001) than the BCL2-negative group (Table 1). The rate of recurrence or death was statistically significantly lower in the BCL2-positive group than in the BCL2-negative group (P < 0.001) (Table 1).
Positive clinicopathological characteristics and a younger age of less than 50 years were shown to be connected with the BCL2-positive group.
The clinical and pathologic findings of the 254 patients with tumor recurrence are included in Table 2. Between the BCL2-positive and BCL2-negative groups, there were statistically significant differences in terms of age, tumor size, histologic grade, stage, ER status, PR status, HER2 status, molecular subtype, adjuvant treatment, time to recurrence, and death rate. The BCL2-positive group with recurrence was significantly associated with younger age (P = 0.013), smaller tumor size (P = 0.009), early-stage disease (P = 0.004), lower histologic grade (P = 0.001), hormone receptor positivity (P < 0.001), HER2 negativity (P < 0.001), higher rate of luminal molecular subtype (P < 0.001), and higher rate of adjuvant endocrine therapy (P < 0.001) than the BCL2-negative group with recurrence (Table 2).
The time to recurrence was significantly longer in the BCL2-positive group than in the BCL2-negative group (47.88 ± 2.66 months vs. 39.2 ± 3.151 months, P = 0.035). Late recurrence after 5 years was 18.7% in the BCL2-negative group and 30.5% in the BCL2-positive group, which was statistically significantly higher in the BCL2-positive group (P = 0.029). Early recurrence within 5 years was 81.3% in the BCL2-negative group and 69.5% in the BCL2-positive group.
Out of 254, a total of 191 patients with recurrence experienced early recurrence (75.2%), and 63 patients had late recurrence (24.8%). In univariate analysis, time to recurrence showed a statistically significant association with tumor size, pathologic stage, histologic grade, ER status, PR status, Ki-67 status, BCL2 expression, molecular subtype, and adjuvant endocrine therapy (Table 3).
Tumor size and BCL2-positive expression were the only independent prognostic markers for late recurrence of breast cancer, according to multivariate survival analysis with Cox’s proportional hazard model (odds ratio [OR], 1.870; 95% confidence interval [CI], 1.098–3.185; P = 0.021 and OR, 2.297; 95% CI, 1.297–4.067; P = 0.004).
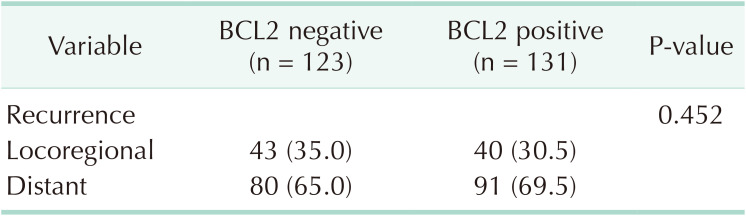
Eighty-three (of the 254 patients 32.7%) experienced locoregional recurrence, and 171 (67.3%) had distant metastasis. The most common site of distant metastasis was the bone (n = 71) followed by the lung (n = 44). The patterns of recurrence in the positive and the negative BCL2 expression groups were not different (Table 4).
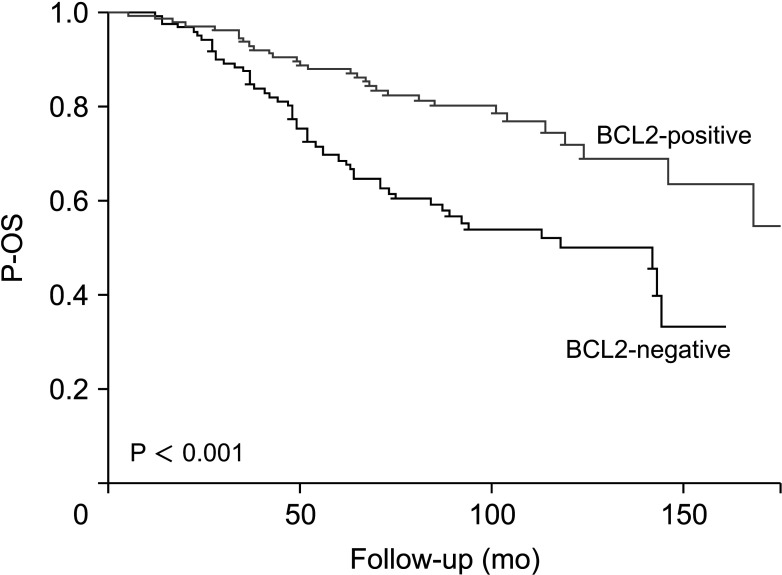
In the 254 patients with recurrence, there were significantly different outcomes for P-OS according to the BCL2 expression status (Fig. 2). Five-year P-OS was 68.4% for BCL2-negative and 87.7% for BCL2-positive (P < 0.001). The BCL2-positive group showed significantly higher rates of P-OS than the BCL2-negative group.
Go to : 
We investigated the role of BCL2 as a prognostic factor for breast cancer. Compared to the BCL2-negative group, the BCL2-positive group showed more favorable clinicopathologic parameters, including tumor size, nodal status, pathologic stage, histologic grade, hormone receptor status, and HER2 status in this study. Our results demonstrated that BCL2-positive group had a higher rate of late breast cancer recurrence than the BCL2-negative group. The postrecurrence survival also showed excellent results in the BCL2-positive group compared to the BCL2-negative group.
BCL2 is a key antiapoptotic or programmed cell death regulatory protein [1315]. BCL2-positivity is expected to correlate with poor prognosis [1617]. In contrast to other solid tumors, BCL2-positive expression in breast cancer is associated with favorable prognosis factors, such as low grade, slow proliferation, and ER positivity [1317]. The mechanism of the paradoxical favorable prognostic effect of BCL2 in breast cancer remains unclear [17]. Many studies have suggested that interactions between BCL2 and ER modulate the prognostic effect [181920].
This study focused on the favorable clinical effects of BCL2 as well as its effects on late recurrence of breast cancer. Many studies have examined the clinical importance of BCL2 in breast cancer [212223]. Although there is still a debate about the paradoxical role of BCL2, most studies concluded that BCL2-positivity predicted a favorable clinical outcome in breast cancer [1321242526]. Similar to our results, several previous studies revealed an association with favorable features in BCL2-positive breast cancer patients and proved BCL2 as a favorable prognostic factor. Callagy et al. [26] evaluated the prognostic potential of 13 biomarkers in 930 breast cancers on a tissue microarray and showed that BCL2 expression had a powerful prognostic impact independent of the Nottingham prognostic index. They also proved the prognostic role of BCL2 in a meta-analysis of 18 studies with 2,285 cases [22]. Dawson et al. [13] performed a meta-analysis of 5 studies with 11,212 cases and concluded that BCL2 was an independent indicator of favorable prognosis for all types of early-stage breast cancer.
Hwang et al. [27] showed that the BCL2-positive group had more favorable features in all clinicopathologic parameters, and all subtypes showed better overall survival (OS) and disease-free survival (DFS) than the BCL2-negative group. In a more recent study, they assessed BCL2 expression levels by IHC using tissue microarrays from 393 breast cancer patients and found that the high-intensity BCL2 expression group had superior DFS compared to the low-intensity BCL2 group. In this study, the favorable prognostic effect of BCL2 expression was detected only in the hormone receptor-positive and HER2-negative groups [12].
In contrast, Honma et al. [21] reported that BCL2 expression in the ER-negative and PR-negative, and triple-negative group without adjuvant therapy, especially in postmenopausal women, had an independent unfavorable prognostic impact. They reported that the favorable prognosis of the BCL2-positive group might be indirectly affected by co-expressed hormone receptors and adjuvant endocrine therapy. Although most research typically established a 10% cut-off value for BCL2-positivity, this study chose a cut-off value of 30%, which may have led to different outcomes.
Mammographic screening has improved early breast cancer identification, which has led to a rise in the number of long-term survivors [28]. To identify individuals with a high risk of late recurrence among long-term survivors of breast cancer and avoid missing the diagnosis of disease recurrence, it is important to understand the factors associated with the risk of late recurrence.
Several studies investigated the predictors of late recurrence of breast cancer, including clinicopathologic parameters and molecular tools. Wangchinda et al. [29] retrospectively analyzed the data of 300 recurrent breast cancer patients and divided them into those whose relapse times were longer or shorter than 5 years. Tumors larger than 2 cm, LN metastasis, and high nuclear grade were associated with early recurrence, whereas hormone receptor positivity and the HER2-negative subtype were related to late recurrence of breast cancer. Pedersen et al. [30] investigated the risk of late recurrence and clinicopathologic features in early breast cancer in patients 10 years or more, up to 32 years after primary breast cancer diagnosis, using the Danish Breast Cancer Group clinical database from 1987 to 2004. Among 36,924 patients, 2,595 developed late recurrence, with the associated features of tumor size greater than 20 mm, LN-positive disease, and hormone receptor positivity. Recurrences continued to occur up to 32 years after primary diagnosis. These results showed that certain clinicopathologic characteristics, including tumor size, nodal status, and histologic grade, had a prognostic impact not only in early recurrence but also in late recurrence of breast cancer.
As these studies showed, the hormone receptor-positive subtype is prone to occur late recurrence of breast cancer, defined as occurring more than 5 years after diagnosis. Delineating the risk of late recurrence in patients with hormone receptor-positive breast cancer and accessing more accurate information to identify who might be spared or benefit from further extended hormone therapy is important because of the side effects and toxicities of the drugs [28].
This is similar to the results of our study, where tumors larger than 2 cm and BCL2-positive expression were related to late breast cancer recurrence (P = 0.021 and P = 0.004), respectively. The treatment guidelines recommend treatment based on risk groups that include tumor size. Thus, patients with large tumors might have received more aggressive treatment at the time of their diagnosis, and the effect of these treatments may be responsible for late breast cancer recurrence rather than early. BCL2 expression is downstream of the estrogen signaling pathway and is highly expressed in hormone-dependent breast cancer. However, lower BCL2 expression is associated with more frequent resistance to endocrine therapy and higher early recurrence rates. BCL2-positive expression is associated with the late recurrence of breast cancer. However, various antibodies are used to assess BCL2 expression, and the cut-off number of positive cells defining a tumor with BCL2 overexpression often varies according to the antibodies, which may lead to biased conclusions [2223]. The cut-off in the number of positive cells defining a tumor with BCL2 overexpression varies from 5% to 50% and were more than 10% in most studies [2223]. Clone 124 is the most commonly used and was used in the majority of studies where BCL2 was an independent predictor of DFS or OS [2223]. In our study, BCL2-positivity was defined as the presence of cytoplasmic staining in >10% of the malignant cells, following the practice used in most previous studies using clone 124.
In our study, BCL2-positive expression was also associated with late recurrence and better OS after recurrence compared to BCL2-negative expression. This suggests that BCL2-positive expression might be a good prognostic factor for the late recurrence of breast cancer.
However, there were some limitations. First, because this study was retrospective, selection bias may have been present. Second, differences according to the degree of BCL2 expression could not be analyzed. However, we obtained standardized BCL2 measurements performed by a single pathologist at a single institution. Detailed clinicopathologic data and long-term follow-up duration are also strengths of our study. Given the size of the sample, there is a chance that subgroup analyses will yield more statistical power.
In conclusion, our results demonstrate that BCL2-positive expression has mostly seen in hormone-dependent breast cancer, and prognosis is better in BCL2-positive patients compared to BCL2-negative patients at late recurrence. This is suggesting that BCL2 expression could be used as a marker to help determine additional adjuvant therapy or extended hormone therapy in hormone-dependent breast cancer.
Go to : 
References
1. Fumagalli C, Barberis M. Breast cancer heterogeneity. Diagnostics (Basel). 2021; 11:1555. PMID: 34573897.
2. Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020; 20:89–106. PMID: 31836838.
3. Leone JP, Vallejo CT, Hassett MJ, Leone J, Graham N, Tayob N, et al. Factors associated with late risks of breast cancer-specific mortality in the SEER registry. Breast Cancer Res Treat. 2021; 189:203–212. PMID: 33893907.
4. Murata T, Jinno H, Takahashi M, Shimoda M, Hayashida T, Kameyama K, et al. Clinicopathologic features of hormone-receptor-positive breast cancer patients with late recurrence. Breast J. 2019; 25:9–15. PMID: 29687661.
5. Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016; 34:927–935. PMID: 26786933.
6. Sestak I, Cuzick J. Markers for the identification of late breast cancer recurrence. Breast Cancer Res. 2015; 17:10. PMID: 25848913.
7. Esserman LJ, Moore DH, Tsing PJ, Chu PW, Yau C, Ozanne E, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011; 129:607–616. PMID: 21597921.
8. Zhang Y, Schnabel CA, Schroeder BE, Jerevall PL, Jankowitz RC, Fornander T, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013; 19:4196–4205. PMID: 23757354.
9. Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013; 14:1067–1076. PMID: 24035531.
10. Lee YJ, Jung SP, Bae JW, Yang SM, You JY, Bae SY. Prognosis according to the timing of recurrence in breast cancer. Ann Surg Treat Res. 2023; 104:1–9. PMID: 36685773.
11. Foldi J, O'Meara T, Marczyk M, Sanft T, Silber A, Pusztai L. defining risk of late recurrence in early-stage estrogen receptor-positive breast cancer: clinical versus molecular tools. J Clin Oncol. 2019; 37:1365–1369. PMID: 30943126.
12. Hwang KT, Kim YA, Kim J, Oh HJ, Park JH, Choi IS, et al. Prognostic influences of BCL1 and BCL2 expression on disease-free survival in breast cancer. Sci Rep. 2021; 11:11942. PMID: 34099764.
13. Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010; 103:668–675. PMID: 20664598.
14. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–2223. PMID: 23917950.
15. Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003; 22:8590–8607. PMID: 14634621.
16. Vogler M. Targeting BCL2-proteins for the treatment of solid tumours. Adv Med. 2014; 2014:943648. PMID: 26556430.
17. Neri A, Marrelli D, Roviello F, DeMarco G, Mariani F, DeStefano A, et al. Bcl-2 expression correlates with lymphovascular invasion and long-term prognosis in breast cancer. Breast Cancer Res Treat. 2006; 99:77–83. PMID: 16541314.
18. Martin LA, Dowsett M. BCL-2: a new therapeutic target in estrogen receptor-positive breast cancer? Cancer Cell. 2013; 24:7–9. PMID: 23845438.
19. Park SH, Kim H, Song BJ. Down regulation of bcl2 expression in invasive ductal carcinomas is both estrogen- and progesterone-receptor dependent and associated with poor prognostic factors. Pathol Oncol Res. 2002; 8:26–30. PMID: 11994759.
20. Teixeira C, Reed JC, Pratt MA. Estrogen promotes chemotherapeut ic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995; 55:3902–3907. PMID: 7641210.
21. Honma N, Horii R, Ito Y, Saji S, Younes M, Iwase T, et al. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer. 2015; 15:698. PMID: 26472348.
22. Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008; 8:153. PMID: 18510726.
23. Yang D, Chen MB, Wang LQ, Yang L, Liu CY, Lu PH. Bcl-2 expression predicts sensitivity to chemotherapy in breast cancer: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2013; 32:105. PMID: 24370277.
24. Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, et al. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994; 86:499–504. PMID: 8133533.
25. van Slooten HJ, Clahsen PC, van Dierendonck JH, Duval C, Pallud C, Mandard AM, et al. Expression of Bcl-2 in node-negative breast cancer is associated with various prognostic factors, but does not predict response to one course of perioperative chemotherapy. Br J Cancer. 1996; 74:78–85. PMID: 8679463.
26. Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Not tingham Prognostic Index. Clin Cancer Res. 2006; 12:2468–2475. PMID: 16638854.
27. Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK, Moon HG, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012; 131:E1109–E1119. PMID: 22418857.
28. Dowling RJ, Kalinsky K, Hayes DF, Bidard FC, Cescon DW, Chandarlapaty S, et al. Toronto workshop on late recurrence in estrogen receptor-positive breast cancer: part 1. Late recurrence: current understanding, clinical considerations. JNCI Cancer Spectr. 2019; 3:pkz050. PMID: 32337479.
29. Wangchinda P, Ithimakin S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J Surg Oncol. 2016; 14:223. PMID: 27557635.
30. Pedersen RN, Esen BÖ, Mellemkjær L, Christiansen P, Ejlertsen B, Lash TL, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. 2022; 114:391–399. PMID: 34747484.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download