METHODS
This single-center, retrospective, observational study analyzed data obtained from the medical records of patients who had explantation of infected EVAR grafts at Asan Medical Center, Seoul, Korea. The study protocol was approved by the Institutional Review Board of Asan Medical Center (No. 2013-1027). The requirement for informed patient consent was waived due to the retrospective design of the study.
Study population
A total of 12 consecutive patients underwent explantation of infected aortic stent grafts following EVAR between January 1, 2010 and December 31, 2019. One patient who had an extraanatomical reconstruction was excluded from the study. This patient previously had a left external iliac limb graft embolization and right-to-left femoral-femoral bypass for the correction of a type III endoleak resulting from a left distal iliac limb migration. After removal of the infected aortic stent graft and axillo-femoral bypass for revascularization, this patient also had an aortic exclusion surgery. Finally, 11 consecutive patients with in situ graft reconstruction after stent graft removal were included in the study.
Diagnosis and preoperative antibiotics
Contrast-enhanced CT scans with or without white blood cell single-photon emission using 99mTc hexamethylpropylene amine were performed. Results from the CT scan were confirmed by culturing and identification of the pathogens from blood and tissue samples of all 11 patients. Broad-spectrum (either intravenous quinolones alone, or vancomycin plus carbapenem) antibiotics were administered before surgery, and antibiotics (based on antibiotic sensitivity tests) were administered for at least 4 weeks postoperatively.
Surgical procedure
Our principles for treating infected aortic disease (including primary infected AAA, infected aortic prosthetic grafts, and infected aortic stent grafts) have been previously reported [
1011] and were also applied in this study. All surgeries were elective, except in 1 case of rupture. The surgical procedures included suprarenal or supraceliac clamping, complete debridement of the infected aneurysm (including the aortic stent graft and perianeurysmal tissue), antibiotic saline irrigation,
in situ interposition using a prosthetic graft or banked allograft, and omental wrapping. The initial clamping location was based on the previous EVAR graft fixation and the presence of calcification. Subsequent clamping, after the removal of the stent graft, was applied infrarenally, based on the proximity of the initial aneurysm to the renal artery. To prevent reinfection, an antibiotic solution (based on antibiotic sensitivity tests) was used for irrigation. Furthermore, omental wrapping was performed retrocolically to fill the dead space and eliminate residual infection.
Primary and secondary outcomes
Primary outcomes were in-hospital complications (including cardiac, renal, and pulmonary complications) and mortality. Renal complications included postsurgical acute kidney injury, which was defined as an increase in serum creatinine (≥150% above the baseline, or ≥0.3 mg/dL within 48 hours after the procedure), Pulmonary complications included X-ray or CT findings of atelectasis or pneumonia, which required prolonged hospital stay for pulmonary rehabilitation. Cardiovascular complications included myocardial infarctions and cerebral strokes.
Secondary outcomes included reinfections and long-term all-cause mortality after discharge from the hospital. Clinical features, blood culture, and CT scan of the aorta were used to confirm reinfections of the newly implanted graft.
Data analysis
For each patient, the following data were recorded: baseline and clinical characteristics, primary pathology of AAA, events following EVAR, time to explantation, apparent symptoms, port of entry, surgical procedure, microorganisms cultured and identified, and clinical outcomes of the study population. Summary statistics were presented as frequencies or percentages for categorical variables and means and standard deviations (SDs) for continuous variables. Long-term overall patient survival rates were analyzed using the Kaplan-Meier survival curves. All analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp.).
Go to :

RESULTS
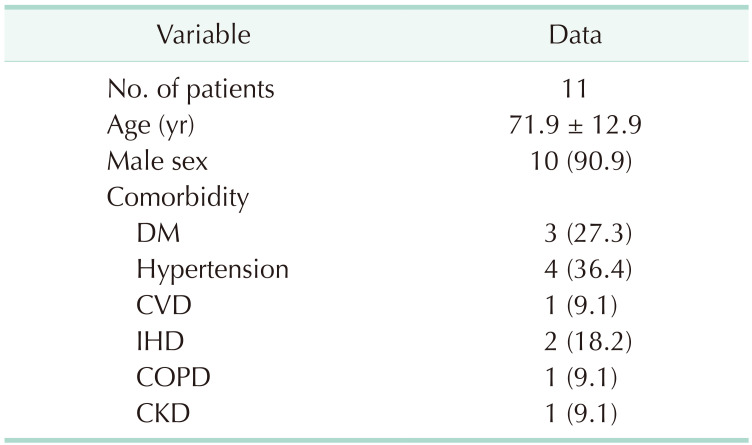
Baseline and comorbidity characteristics of the patients (10 males and 1 female; mean age, 71.9 years) are shown in
Table 1. The mean period between EVAR for AAA and explantation was 33.9 months (SD, ±39.57).
Table 1
Patients’ demographic and clinical characteristics

During the study period spanning between January 1, 2010 and December 31, 2019, 12 patients underwent explantation of infected EVAR grafts. Among these patients, 3 had previously undergone EVAR at other hospitals, and 2 had undergone EVAR in 2005 and 2018, respectively. We analyzed 353 EVAR procedures that were performed between January 1, 2008 and December 31, 2015 to determine the incidence of EVAR graft infection at our center. Of these procedures, 7 were EVAR graft explantation because of infected stent grafts. Therefore, the long-term incidence of an endograft infection after EVAR at our center was found to be 2% over a period of more than 8 years.
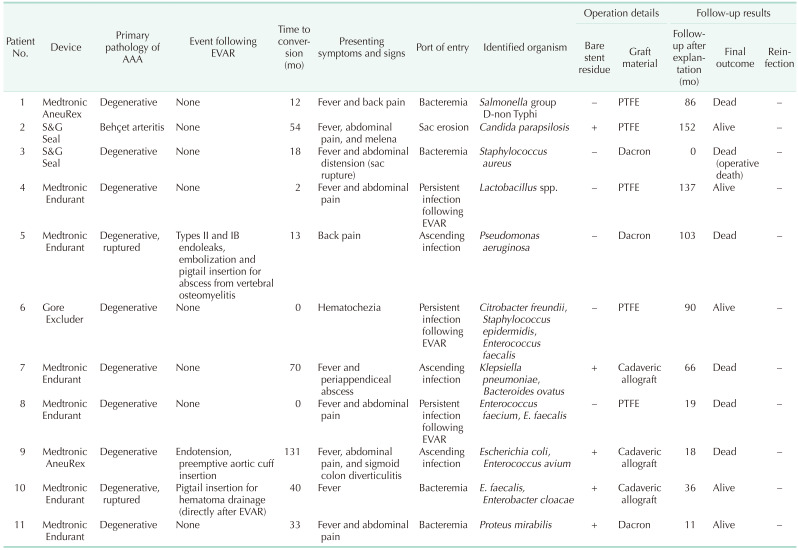
One patient had Behçet arteritis, and the original pathologies of all other patients were degenerative (including 2 with ruptured AAAs). Profiles of all the patients are shown in
Table 2. The average number of secondary interventions (after EVAR and before explantation of the infected graft) was 0.36 (SD, ±0.67). Fever was the most common presenting symptom (81.8%). Not surprisingly, all patients presented with septic conditions and positive blood or tissue cultures (the causative organisms were found in the blood and aortic tissue of 5 and 9 patients, respectively). Surgery was performed as previously described in a semi-elective manner, after administration of broad-spectrum or sensitive antibiotics.
Table 2
Patients’ profiles

For all patients, with the exception one with a ruptured AAA, the operative risk was evaluated before surgery using echocardiography, electrocardiography, and chest radiographs. The mean risk (based on the American Society of Anesthesiologists classification) was 2.5. Additionally, 9 patients had the stent graft with suprarenal fixation barbs and 2 patients had barbs just below the renal artery ostium. Therefore, suprarenal or supraceliac aortic clamping was used before the removal of the infected stent graft. Total explantation of both the fabric portion and the bare stent was performed in 6 patients, and complete removal of only the fabric portion was performed in 5 patients.
Fig. 1A shows the completely removed stent graft (Gore Excluder, W. L. Gore & Associates, Inc.). For
in situ reconstruction after stent graft removal, banked cadaveric allografts were used in 3 patients, and prosthetic grafts were used in 8 patients (5 with polytetrafluoroethylene and 3 with Dacron). For exceptional cases (such as perforated sigmoid colon diverticulitis and periappendiceal abscess) with intraabdominal sepsis, cadaveric allografts were used. The prosthetic grafts or allografts were wrapped with omentum in all cases.
Fig. 1B, the postoperative CT image of a patient who underwent an
in situ reconstruction after explantation of an infected EVAR graft, shows a patent Dacron graft that has been wrapped with omentum.
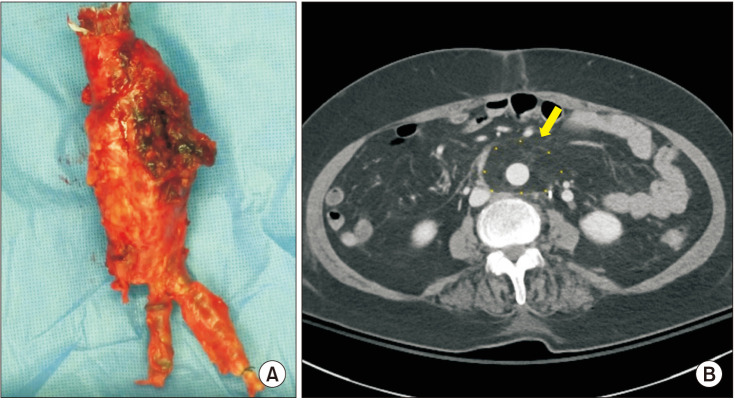 | Fig. 1(A) Surgical photo of the explanted stent graft (Gore Excluder, W. L. Gore & Associates, Inc.) of a patient who had persistent infection after endovascular aneurysm repair (EVAR) with an aortoduodenal fistula. The stent graft was removed completely. (B) Postoperative CT image of the patient who underwent in situ reconstruction after explantation of the infected EVAR graft, showing the patent Dacron graft with omental wrapping (yellow arrow).
|
Based on the possible route of entry of the microorganisms, 4 mechanisms were proposed for aortic stent graft infection in the 11 patients: 4 cases of bacteremia from systemic infection; 3 cases of persistent infection following EVAR of primary infected AAA; 3 cases of ascending infection from an adjacent abscess (1 periappendiceal abscess, 1 vertebral osteomyelitis [OM], and 1 sigmoid colon diverticulitis), and 1 aneurysmal sac erosion resulting in an aortoenteric fistula.
Pathogens and antibiotics
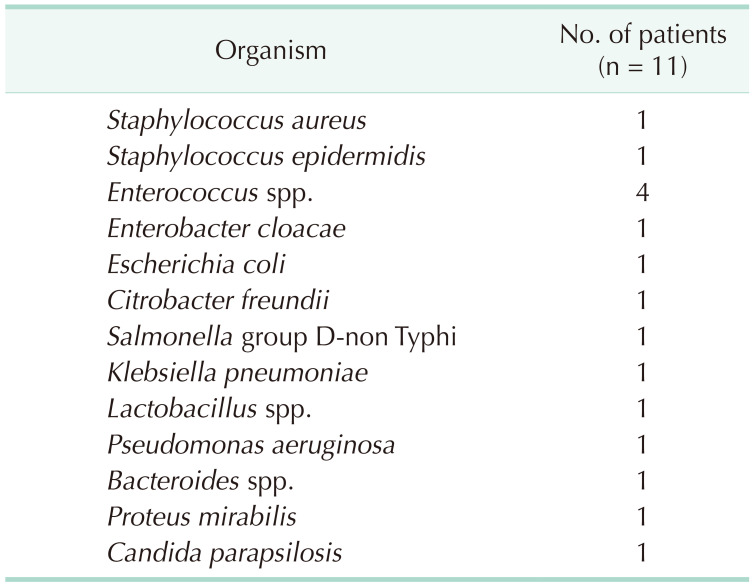
Bacteria were cultured from all the patients and identified using standard protocols. Interestingly,
Enterococcus sp. was cultured and identified more frequently than other pathogenic bacteria (
Table 3). Notably, more than 2 types of bacteria were identified in the endografts or the aortic aneurysms of 4 patients. Broad-spectrum antibiotics were administered to all patients preoperatively (mean, 5.1 days; range, 1–11 days) until the culture results were confirmed. Postoperatively, antibiotics were administered based on antibiotic sensitivity tests (mean, 192.8 days; range, 40–800 days, except for the single in-hospital mortality case).
Table 3
Bacteria cultured from blood or tissue samples

Clinical outcomes
For the 11 patients who underwent explantation, the mean operative time was 433 minutes (range, 300–684 minutes; SD, ±108.21), and the median duration of the postoperative intensive care unit stay was 8 days (range, 2–62 days). Perioperative complications after explantation of the infected grafts were noted in 4 patients (36.4%) (
Table 4). One patient (who had a methicillin-sensitive
Staphylococcus aureus bacteremia) died after the surgery because of a ruptured aneurysm. Although negative conversion of bacteremia was confirmed in this patient after culturing, aortic rupture occurred 10 days after the surgery. An exploration was performed to identify the source of the bleeding; however, failure to control the bleeding occurred. The patient was confirmed to have died of excessive bleeding after an aortic wall injury due to bare stent retrieval by force, and there was no evidence of persistent infection. Furthermore, 1 patient was reoperated for an aneurysm in the proximal anastomosis site 3 months after explantation. The prosthetic graft was removed, the proximal aorta was excluded, and an axillobifemoral bypass was performed. No bacteria were identified in the culture.
Table 4
Perioperative complications after explantation


For the 10 patients, the 1-year and 5-year overall survival rates were 90.9% and 72.7%, respectively (
Fig. 2). There was no evidence of reinfection in the surviving patients, regardless of the graft materials used for
in situ reconstruction, residual bare stents, or pathogens identified. The mean follow-up duration after explantation was 65.27 months (SD, ±52.51).
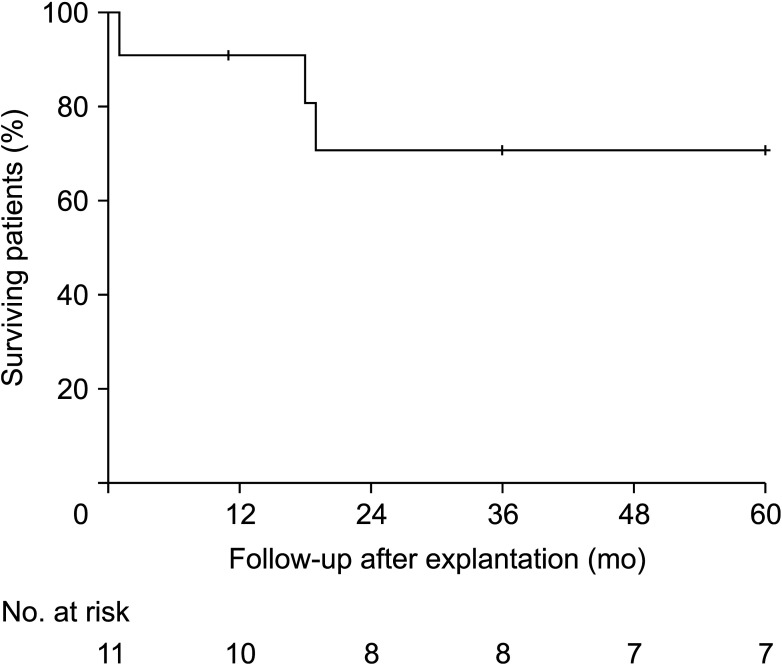 | Fig. 2The Kaplan-Meier estimate of overall 5-year survival rates among 11 patients with in situ reconstruction after explantation of infected stent grafts.
|
Go to :

DISCUSSION
Although rare, stent graft infection can be a devastating potential complication after EVAR. Our study identified 4 mechanisms of graft infection in which bacteremia from systemic infection was the most common, followed by persistent infection following EVAR for primary infected AAA, ascending infection from an adjacent abscess, and aneurysmal sac erosion resulting in an aortoenteric fistula. Various pathogens were identified in 4 patients whose graft infection mechanism was bacteremia. Although
S. aureus is most commonly reported in aortic graft infection [
12], only 1 patient in our study had
S. aureus bacteremia. Nontyphoidal strains of
Salmonella, which are common causes of aortic infections in Asia, were identified in the blood of 1 patient, [
13].
Proteus mirabilis, which is a common cause of urinary tract infections, was identified in the blood of 1 patient with concomitant urosepsis. Persistent infection after EVAR of primary infected AAA, another mechanism of graft infection, caused explantation in 3 patients. These patients were immediately transferred from other hospitals to ours for further management of persistent infections.
According to recent studies, EVAR is an acceptable alternative for primary infected aortic aneurysms [
1415]. However, persistent or recurrent infections can occur after EVAR [
14]. Moreover, aortoduodenal fistula, a rare but dangerous complication of EVAR, can occur secondary to infection [
16]. In our study, aortoduodenal fistulas were identified in all 3 patients with persistent infections of their aortic aneurysms after EVAR. One patient showed no evidence of an aortoenteric fistula at the time of EVAR. However, a secondary aortoenteric fistula may have developed in this patient after EVAR of the infected AAA (time from EVAR to explantation, 75 days). Additionally, 2 patients presented with gastrointestinal bleeding at the time of EVAR. These patients may have had primary infected AAAs with aortoenteric fistulas before EVAR (mean time from EVAR to explantation, 24 days). A study from Thailand showed that the outcomes of EVAR for infected aortic aneurysms were poorer with fistulous complications [
15]. In their study, patients who presented with infected aortic aneurysms and fistulous complications had an overall in-hospital mortality of 60% after EVAR [
15]. A 43% rate of persistent sepsis and/or recurrence of aortoenteric fistulas after EVAR has also been reported [
17]. Our results, as well as those of previous studies, suggest that EVAR of infected aortic aneurysms with suspected fistulous complications must be reconsidered due to the high probability of persistent infection.
Ascending infection from an adjacent abscess was yet another mechanism of graft infection that was found in 3 patients. An ascending infection from pigtail drainage of an abscess arising from vertebral OM was identified in 1 patient who had undergone EVAR for a ruptured AAA. A month after EVAR, the abscess arising from chronic vertebral OM (L4–5 level) was drained via a pigtail catheter and maintained for 5 months with antibiotics. The stent graft was removed due to persistent chronic infection 6 months after removal of the pigtail catheter. One patient with a known left internal iliac artery (IIA) aneurysm developed a fistula between the left IIA aneurysm and an abscess from a sigmoid colon diverticulitis. The patient had presented with a left IIA aneurysm before EVAR and underwent embolization for exclusion of the left IIA aneurysm during EVAR. However, exclusion of the IIA aneurysm failed. The size of the left IIA aneurysm increased progressively and resulted in a left IIA aneurysm-sigmoid colon fistula when the diverticulitis perforated 131 months after the EVAR. Moreover, another patient with an ascending infection presented with a fistula between the AAA sac and a periappendiceal abscess.
Aortoenteric fistula arising from sac erosion was the fourth mechanism of graft infection identified in a single patient who also had Behçet disease. An aortoduodenal fistula was identified in this patient who had initially undergone a patchy angioplasty for saccular AAA, and subsequently underwent EVAR for a recurrent aneurysm 60 months after the first procedure.
When an infected aortic stent graft is identified, the provision of adequate antibiotics and maintaining a patient’s stable condition should be prioritized in order to lower operative risks. An infected aortic stent graft is prone to sac rupture. Therefore, early surgery and close observation are recommended [
18], as the prognosis is poor in cases of sac rupture. In our study, the patient who presented with sac rupture before explantation died during the postoperative period. Some researchers have advocated surgical protocols for infected AAA [
913]. We have previously reported our principles for treating infected aortic disease, including primary infected AAA, infected aortic prosthetic grafts, and infected aortic stent grafts [
1011]. These studies showed favorable outcomes for
in situ prosthetic graft bypasses with omental wrappings [
10111920]. We applied the same surgical principles to all cases of infected stent grafts in this study. In our surgical method, stent graft removal and debridement must be sequentially performed. Furthermore, explantation should be performed with caution because the infected stent graft is in the middle of liquid pus and/or the infected thrombus within the sac. Therefore, it can be easily dislodged, leading to massive bleeding. In cases of explantation of the infected stent graft, the infrarenal procedure is changed to the suprarenal or supraceliac procedure to procure healthy tissue for anastomosis. Removal of the suprarenal barb fixations is the most challenging procedure during explantation of the EVAR device [
21] since the risk of proximal aortic wall damage is high [
22]. After EVAR, the bare stent portion fuses with the aortic wall over time, and removal of the barbs becomes technically difficult. Several reports describe a method for removing the suprarenal barbs effectively [
2324]. However, to prevent aortic wall damage, the bare stents should be removed one-by-one after separating the fabric portion using a cutter. Aortic wall injury may occur while removing the barbs forcibly in this method. Notably, the only mortality in our study resulted from the rupture of a proximal aortic wall pseudoaneurysm that had occurred during bare stent removal by force. The barbs, which are a foreign body, should be removed completely to reduce the chance of reinfection. However, the chance of an aortic wall injury increases with forced removal.
Our study showed no reinfection in 5 cases with a bare stent residue. The bare portion of the stent is known to cause fewer foreign body reactions than the fabric portion. Therefore, we suggest that leaving behind the bare stent residue in situ is a better strategy than forced removal, which might result in aortic wall injury. After graft removal is complete, the proximal aortic neck is managed, the clamp is distally moved to the suprarenal or infrarenal aorta, and complete debridement is performed.
Currently, there is controversy regarding the optimal reconstruction method for an infected aorta. Extra-anatomic reconstruction may be favorable in the presence of an aortoenteric fistula [
25]. However, several reports, including our previous study, showed that
in situ reconstruction with a prosthetic graft result in good patency and a low rate of procedure-related deaths or major complications [
1126]. In our previous study, all
in situ-replaced grafts for the prosthetic aortic graft-enteric fistulas became reinfected during the follow-up period [
11]. However,
in situ reconstruction for aortoenteric fistulas in primary infected AAA and infected stent graft were managed without late reinfection during follow-up [
11]. Since an extra-anatomic bypass has several disadvantages when compared to an
in situ bypass, including the risk of stump rupture and a high rate of long-term graft occlusion [
7], we performed
in situ reconstructions in all 6 patients with aortoenteric fistulae, and there was no reinfection.
Regarding graft materials used for aortic reconstruction, we previously reported the safety of prosthetic grafts, even in the absence of antibiotic soaking [
1119]. Some authors reported that reconstruction with an allograft or autologous femoral vein has the advantage of reduced rates of infection. However, aortic reconstruction with an allograft must be performed only at a hospital with a tissue bank, and where commercial products have limited availability and/or costlier [
272829]. Additionally, reconstruction with an autologous femoral vein has some disadvantages. Most importantly, it is a very complex and time-consuming procedure. According to a recent study, the mean operating time for this surgery was 645 minutes, which was much longer than the time reported in this present study (433 minutes) [
28]. In our study, banked allograft was used only in the case with intraabdominal sepsis because of limited availability. Similarly, a previous study showed that reinfection rates were similar for different materials used in reconstructions [
5].
One of the most important processes for preventing reinfection is the omental wrapping of the newly implanted graft after extensive debridement of the infected tissue. The omentum has rich blood circulation and lymphoid tissues with a high absorptive capacity that facilitates the clearance of bacteria [
30]. Omental wrapping of the graft induces the absorption of necrotic tissues and fills dead space, thereby controlling residual infections.
The optimal duration of antibiotic treatment after explantation of an infected stent graft is controversial [
7]. We administered antibiotics for at least 4 weeks postoperatively and considered discontinuing the antibiotics if there was no clinical or laboratory evidence of an active infection. In our study, lifelong antibiotics were not required in any patient.
This study had several limitations. First, this was a single-center study, and the retrospective analysis has its inherent limitations. Additionally, potential selection and information biases may exist on the part of physicians or patients. The small sample size limits the generalization of the results, and the study population consisted only of patients of Asian descent.
In conclusion, choosing a proper surgical technique is important, and proximal aortic wall damage should be avoided during bare stent removal, even when using suprarenal or supraceliac clamps. Moreover, leaving bare stents in the aortic wall did not result in reinfection in this study, and the use of in situ reconstruction after massive antibiotic irrigation followed by omental wrapping was not associated with reinfection. Therefore, we suggest that in situ reconstruction after explantation of infected aortic stent grafts is comparable with the procedures carried by previous studies, regardless of the graft material and bare stent residue. Furthermore, without lifelong antibiotics, no reinfection occurred in our study.
Go to :






 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download