METHODS
This study was approved by the Institutional Review Board of Samsung Medical Center (No. 2021-10-043). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
Patients and data
The hepatic venous territory mapping program was started in January 2019. To compare whether there was any difference before and after utilizing hepatic venous territory mapping, patients who underwent LDLT using right liver graft before the program were also included. Therefore, patients who underwent LDLT using right liver graft during the period of January 2017 to July 2021 at our center were reviewed for study inclusion. Data on patient demographics, LT-related and recovery-related data were reviewed.
Data regarding venous reconstruction were also collected. Whether right inferior hepatic vein and V5/V8 branches of the middle hepatic vein were reconstructed was reviewed, as well as the length of the vein opening measured during back-table procedure. The types of graft used for V5/V8 reconstruction were collected. At 2 weeks after LT, routine CT was taken and the patency of the reconstructed veins was reviewed.
Hepatic venous territory mapping
Preoperatively, the proportion of volume of each hepatic vein branch was measured using the Volume viewer application in the AW server 3.2 (GE Healthcare). The veins included were right hepatic vein, inferior hepatic vein, and V5 and V8 branches draining into the middle hepatic vein. Both the volume (cm
3) and proportion (%) of the territory compared to right hemi-liver were calculated (
Fig. 1). Whether to reconstruct the middle hepatic vein territories or not was decided by the surgeons based on the mapping data and the graft’s finding during perfusion. Cryopreserved iliac vessels were used for reconstruction of the middle hepatic vein branches. When available, iliac vein grafts with proper size and length were preferred. However, iliac artery grafts were frequently used due to shortage of proper cryopreserved grafts.
 | Fig. 1Technical steps for hepatic venous territory mapping. Using the Volume viewer in the AW server (GE Healthcare), (A) hepatic parenchyma is 3-dimensionally mapped and volume is calculated excluding the volume of major vessels. (B) As the hepatic venous branches are labeled, (C) it is combined with the parenchyma to divide the districts based on the venous territories. IHV, inferior hepatic vein; RHV, right hepatic vein.
|
Hepatic venous outflow reconstruction
The decision to reconstruct the venous outflow was decided both preoperatively and intraoperatively. When the outflow volume is large and proportion is expected to exceed 20% of graft volume, reconstruction was usually decided preoperatively. Even when the outflow was less than 20%, the decision to perform reconstruction was made intraoperatively based on observation of outflow during back-table preservation solution perfusion. Cryopreserved vessels were used with the adequate diameter and length based on measurement. Reconstructed graft was anastomosed to the inferior vena cava. Iliac vein grafts were preferred compared to arteries. When the outflow reconstruction was obstructed based on Doppler ultrasonography or computed tomographic scan, thrombectomy or thrombolysis was decided by judging the clinical impact on the recipient.
Statistical analysis
The primary goal of this study was to analyze whether a hepatic venous mapping program added benefit to the clinical outcome of LDLT patients. To examine this, we compared the 2 groups with or without hepatic venous mapping and further performed survival analyses for graft and overall patient survival including other potential risk factors. Kaplan-Meier survival using the log-rank test and multivariable Cox proportional hazard model was used for graft and overall survival analysis.
The secondary goal was to analyze the factors related to successful venous reconstruction using the data acquired through hepatic venous mapping. For this, only the patients with hepatic venous mapping were analyzed. To set a cutoff point for volume and percentages of the vein branches, receiver operating characteristics analysis with Youden index was used. For analyzing risk factors for reconstructed vein occlusion, multivariable logistic regression analyses were performed. Since the type of venous reconstruction varied among patients, the analyses were performed on different subgroups of patients which were considered adequate for analysis.
Go to :

RESULTS
Patient group comparison with or without hepatic venous mapping
During the study period, a total of 445 patients received LDLT using right hemi-liver. Hepatic venous territory mapping started in January 2019 and a total of 213 patients’ venous territories were preoperatively measured. The median right hemi-liver volume based on the mapping data was 865 cm3 (interquartile range [IQR], 716–1,010 cm3). The median volume of right hepatic vein territory was the largest (380 cm3; IQR, 237–535 cm3) while V8 territory was the smallest (122 cm3; IQR, 79–171 cm3).
Table 1 summarized the comparison between the 2 groups. Since hepatic venous mapping was initiated in 2019, there was a significant difference in the year of LT between the 2 groups (P < 0.001). Regarding inferior hepatic vein, there were no differences in reconstruction rate (26.5% in no mapping
vs. 30.2% in mapping, P = 0.398), patency rate (91.7% in no mapping
vs. 96.8% in mapping, P = 0.425), and median length of vein opening (21.0 mm in no mapping
vs. 19.0 mm in mapping, P = 0.646). Regarding middle hepatic vein, while reconstruction rate was higher in patients with hepatic venous mapping (68.7%) compared to patients without mapping (55.8%, P = 0.005), the patency rates were similar between the 2 groups (63.5% in no mapping
vs. 52.1% in mapping, P = 0.059). There were no statistical differences between the 2 groups regarding the median length of vein opening for both V5 (11.0 mm in no mapping
vs. 12.0 mm in mapping, P = 0.053) and V8 (10.0 mm in no mapping
vs. 10.0 mm in mapping, P = 0.065).
Table 1
Comparison between patients with or without hepatic venous volume mapping


Primary endpoint: graft survival and overall survival
Multivariable Cox analyses for graft survival and overall survival are summarized in
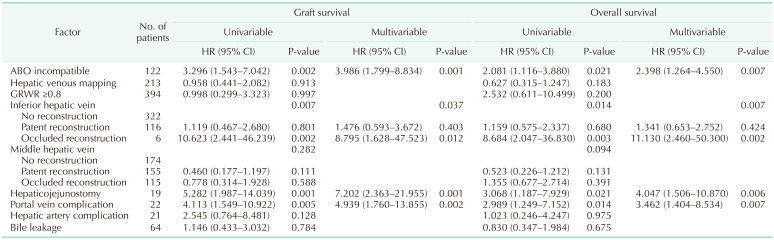
Table 2. While patency of middle hepatic vein reconstruction was not related to both graft and overall survival, patency of inferior hepatic vein reconstruction showed a significant relationship both for graft survival (P = 0.037) and overall survival (P = 0.007). While patent reconstructed inferior hepatic vein showed similar graft (hazard ratio [HR], 1.476; 95% CI, 0.593–3.672; P = 0.403) and overall survival (HR, 1.341; 95% CI, 0.653–2.752; P = 0.424) compared to patients with no inferior hepatic vein reconstruction, patients with occluded inferior hepatic vein reconstruction showed worse graft (HR, 8.795; 95% CI, 1.628–47.523; P = 0.012) and overall survival (HR, 11.130; 95% CI, 2.460–50.300; P = 0.002) compared to no reconstruction group. Other factors related to graft survival were ABO incompatibility (HR, 3.986; 95% CI, 1.799–8.834; P = 0.001), hepaticojejunostomy (HR, 7.202; 95% CI, 2.363–21.955; P = 0.001), and portal vein complication (HR, 4.939; 95% CI, 1.760–13.855; P = 0.002). Other variables significantly related to overall survival were ABO incompatibility (HR, 2.398; 95% CI, 1.264–4.550; P = 0.007), hepaticojejunostomy (HR, 4.047; 95% CI, 1.506–10.870; P = 0.006) and portal vein complication (HR, 3.462; 95% CI, 1.404–8.534; P = 0.007). The graft survival and overall survival curves according to the patency of inferior hepatic vein reconstruction and middle hepatic vein reconstruction are presented in
Figs. 2 and
3.
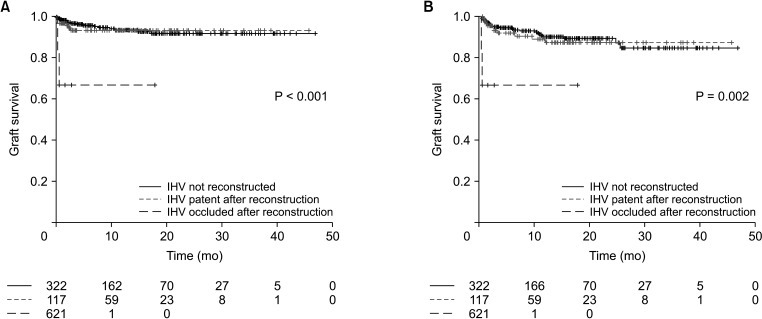 | Fig. 2Kaplan-Meier (A) graft survival and (B) overall survival curves according to the status of inferior hepatic vein (IHV) using right hemi-liver in living donor liver transplantation.
|
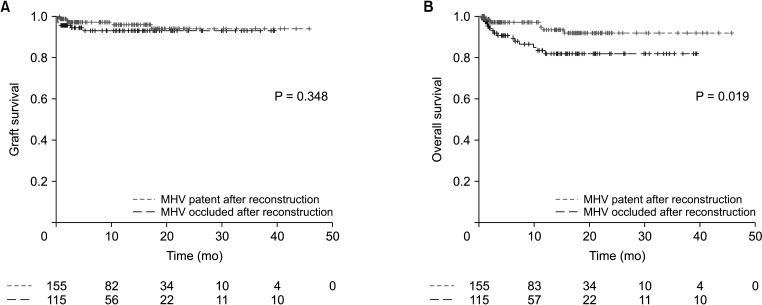 | Fig. 3Kaplan-Meier (A) graft survival and (B) overall survival curves according to the status of reconstructed middle hepatic vein (MHV) in a subgroup of patients with MHV reconstruction.
|
Table 2
Multivariable Cox regression analyzing potential risk factors related to graft survival and overall survival

After excluding patients without middle hepatic vein reconstruction, subgroup analysis on patients with middle hepatic vein reconstruction was performed.
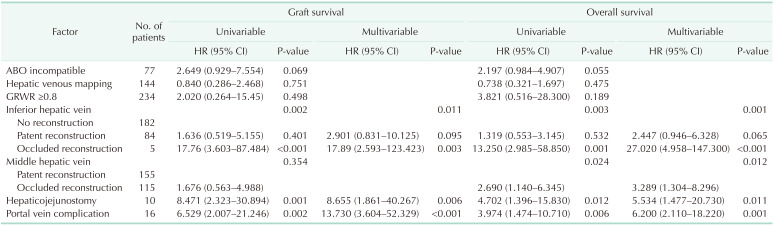
Table 3 summarizes the results of graft survival and overall survival analyses. While patency of middle hepatic vein was not significant for graft survival, occlusion of reconstructed middle hepatic vein showed significantly increased risk on overall survival (HR, 3.289; 95% CI, 1.304–8.296; P = 0.012) compared to patent reconstructed middle hepatic vein.
Table 3
Multivariable Cox regression analyzing potential risk factors related to graft survival and overall survival in a subgroup whose middle hepatic vein territories were reconstructed

Secondary endpoint: factors related to patency of reconstructed vein
Based on the data acquired by hepatic venous territory mapping, comparison of territory volume and percentages among right lobe of specific venous branches between patent group and occluded group were performed (
Table 4).
Table 4
Volume of venous territory according to the patency of reconstructed vein
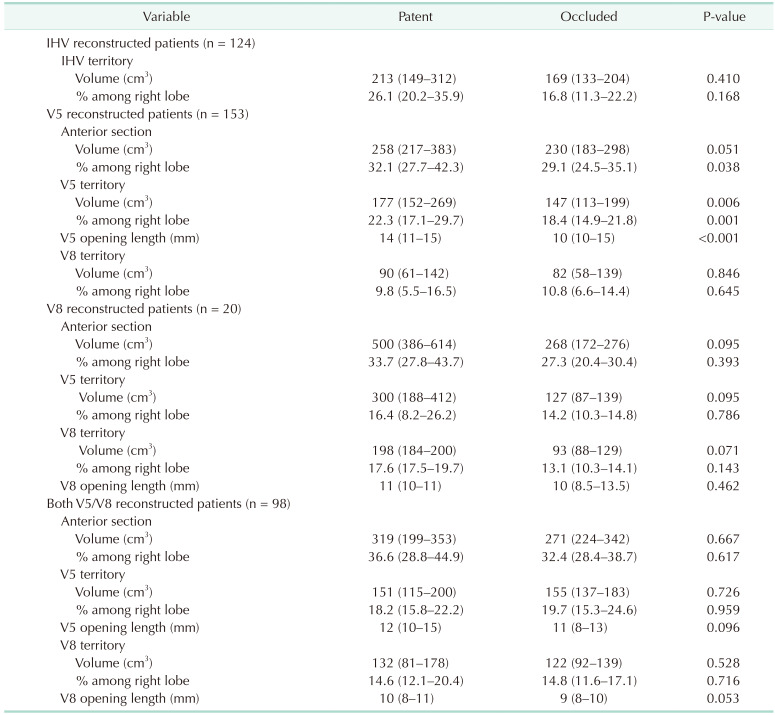

Among, V5 reconstructed patients (n = 153), median percentage of anterior section was higher in the patent group (32.1%; IQR, 27.7%–42.3%) compared to the occluded group (29.1%; IQR, 24.5%–35.1%, P = 0.038). Median volume (177 cm3; IQR, 152–269 cm3) and percentage (22.3%, IQR, 17.1%–29.7%) of V5 territory in patent group were higher than the volume (147 cm3; IQR, 113–199 cm3; P = 0.006)) and percentage (18.4%; IQR, 14.9%–21.8%, P = 0.001) of the occluded group. Median length of the V5 opening was longer in the patent group (14 mm; IQR, 11–15 mm) compared to the occluded group (10 mm; IQR, 10–15 mm; P < 0.001).
To set a cutoff point, Youden’s index in receiver operating characteristics were performed on related variables. Among patients who underwent middle hepatic vein territory reconstruction, length of V5 opening (area under the curve [AUC], 0.639; 95% CI, 0.545–0.732; P = 0.005), volume of V5 territory (AUC, 0.604; 95% CI, 0.511–0.697; P = 0.032), percentage of V5 territory (AUC, 0.621; 95% CI, 0.530–0.712; P = 0.012), length of V8 opening (AUC, 0.523; 95% CI, 0.363–0.683; P = 0.774), volume of V8 territory (AUC, 0.572; 95% CI, 0.476–0.669; P = 0.145), percentage of V8 territory (AUC, 0.564; 95% CI, 0.470–0.658, P = 0.186), volume of right anterior section (AUC, 0.616; 95% CI, 0.521–0.712, P = 0.020), and percentage of right anterior section (AUC, 0.635; 95% CI, 0.545–0.726, P = 0.005) were analyzed. V5 opening of 10 mm (sensitivity, 65.8%; specificity, 57.8%), V5 volume of 150 cm3 (sensitivity, 67.6%; specificity, 51.5%), V5 percentage of 20% (sensitivity, 49.3%; specificity, 73.9%), right anterior section volume of 300 cm3 (sensitivity, 52.2%; specificity, 75.4%), and right anterior section percentage of 35% (sensitivity, 52.0%; specificity, 75.4%) were set as optimal cutoff point.
Among patients who underwent reconstruction of only V5 vein opening, V5 opening length of 10 mm (AUC, 0.721; 95% CI, 0.606–0.835; P = 0.001/10 mm sensitivity, 79.4%; specificity; 64.4%), V5 volume of 150 cm3 (AUC, 0.680; 95% CI, 0.562–0.798; P = 0.006/sensitivity, 79.4%; specificity, 53.3%), V5 percentage of 20% (AUC, 0.716; 95% CI, 0.599–0.833; P = 0.001/sensitivity, 64.7%; specificity, 71.1%) were suitable as a cutoff point.
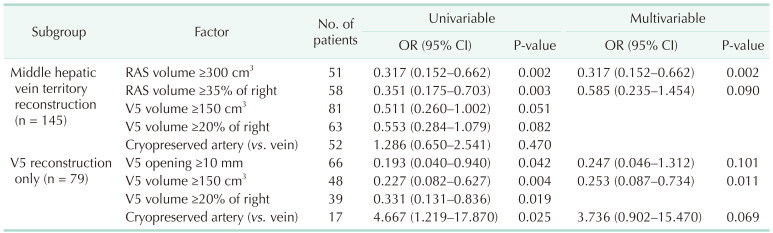
Table 5 summarizes the multivariable logistic regression analysis for potential risk factors of vein occlusion on different subgroups of patients with middle hepatic vein reconstruction and patients with V5 reconstruction. In patients comprising any reconstruction of the middle hepatic vein territory (n = 145), right anterior section volume of ≥300 cm
3 was significantly protective for vein occlusion (OR, 0.317; 95% CI, 0.152–0.662; P = 0.002). In patients with V5 reconstruction (n = 79), V5 volume ≥150 cm
3 was protective for vein occlusion (OR, 0.253; 95% CI, 0.087–0.734; P = 0.011).
Table 5
Multivariable logistic regression analyzing potential factors related to middle hepatic vein graft occlusion in different subgroups with hepatic venous territory mapping

Risk factor analyses for inferior hepatic vein occlusion and V8 were impossible to perform since vein occlusion rate was only 3.1% (2 of 64) in inferior hepatic vein and solitary V8 reconstruction was performed in only 8 patients.
Go to :

DISCUSSION
LDLT has become the major surgical method for patients with end-stage liver disease or malignancy such as hepatocellular carcinoma in regions where deceased donor pool is insufficient to fulfill the need. While whole liver can be used in deceased donor LT, LDLT only allows partial liver. Right hemi-liver which is frequently used for LDLT, usually does not include the main trunk of the middle hepatic vein. Therefore, venous outflow of the middle hepatic vein territory should be reconstructed when the proportion of middle hepatic vein territory is relatively large [
7]. This is not only important for the graft survival but also for lowering the risk of cancer recurrence. There have been studies reporting increased recurrence of malignancy with graft congestion, which may be due to the pro-inflammatory condition developed by ischemia-reperfusion injury [
489]. Although reconstructing the venous outflow when using right hemi-liver is a vital process, it is important to selectively reconstruct major outflow branches since small venous branches usually occlude even when they are reconstructed. However, there have been no studies reporting objective data for venous outflow reconstruction in LDLT using right hemi-liver. The surgeon’s decision to reconstruct or ligate the venous branches is determined based on their experience. In this study, we measured the volumes of the liver parenchyma according to the territory of the venous branches. Based on the volumetric data, we analyzed which factors were related to the venous outflow occlusion.
The first finding which compared before and after the hepatic venous territory mapping showed that middle hepatic vein reconstruction rate had increased after the program. While 55.8% of middle hepatic vein territories were reconstructed before the program, 68.7% of the middle hepatic vein territories were reconstructed after the program (P = 0.005). However, although there was no statistical difference, the patency rate of the middle hepatic vein was 52.1% in patients with hepatic venous territory mapping, while that before the program was 63.5% (P = 0.059). The finding shows that due to the objective data presented preoperatively, there was a tendency to reconstruct the middle hepatic vein territory more frequently than before. However, the patency rate shows that the outcome, whether the outflow is occluded or not, has not changed compared to previous rates. Therefore, it can be assumed that volumetric assessment of hepatic venous territory can influence the decision-making for outflow reconstruction. This can be especially useful for surgeons with limited experience. To increase the patency rate of reconstructed venous structures, surgical strategies including technique, graft type, and surgeons’ learning curve should be considered. However, during the study period, reconstruction strategies were maintained without change. This may be the reason for the similar outcome before and after the program.
The multivariable Cox analyses showed the importance of venous outflow reconstruction; especially for inferior hepatic vein, which is reconstructed in 27.8% of cases (124 of 445 in total) and can be a determinant for graft survival. While patent reconstructed inferior hepatic vein showed similar graft survival (HR, 1.476; 95% CI, 0.593–3.672; P = 0.403) and overall survival (HR, 1.341; 95% CI, 0.653–2.752; P = 0.424) compared to patients who did not require inferior hepatic vein reconstruction, occluded group showed worse graft survival (HR, 8.795; 95% CI, 1.628–47.523; P = 0.012) and overall survival (HR, 11.130; 95% CI, 2.460–50.300, P = 0.002). On the other hand, the importance of middle hepatic vein patency was relatively lower compared to inferior hepatic vein. In a subgroup of patients with middle hepatic vein reconstruction, middle hepatic vein occlusion showed significant risk for patient survival (HR, 27.020; 95% CI, 4.958–147.300; P < 0.001). While middle hepatic vein reconstruction is not always necessary, it should stay patent when it is considered large enough to be reconstructed.
Only half of the study subjects underwent hepatic venous territory volume measurement. The reason we included 232 patients who did not undergo volume measurement was to analyze whether the program actually changed the outcome of our LDLT program. As presented in
Table 2 and
3, the program itself did not significantly influence the outcome, which was set as graft survival and overall survival. Nevertheless, the surgical team could make decisions based on objective data; and, furthermore, we could design a study to analyze factors related to venous outflow occlusion.
Table 5 shows that the volume of the territories itself is important for the patency of the reconstructed venous outflow. When middle hepatic vein territories are reconstructed, the right anterior section volume measured ≥300 cm
3 was protective for vein occlusion. When only V5 branch was reconstructed, V5 volume measured ≥150 cm
3 and was protective against vein occlusion. Although we did not measure the venous outflow, the amount of blood flow proportionately increased with hepatic volume. In fact, the volumetric territory, the percentage of the territory within the right lobe, and the length of the venous opening should proportionately increase or decrease with each other. Among them, the volume itself seems to be the most matched variable to predict the future of the reconstructed venous outflow. When hepatic venous outflow territory was not measured, length of the venous opening itself can be a key indicator for predicting the destiny of venous outflow. The multivariable logistic regression analyses were performed in 145 patients whose middle hepatic vein territories were reconstructed and 79 patients with V5 reconstruction. When we widen the scope to patients without hepatic venous mapping, the discriminating power of the length of venous opening increases. In 154 patients who underwent V5 reconstruction, length of V5 opening showed AUC of 0.677 (95% CI, 0.591–0.762; P < 0.001), and 10 mm as a cutoff showed sensitivity of 80.5% and specificity of 55.3%.
Our center used cryopreserved iliac vessels for venous reconstruction of middle hepatic vein. While iliac veins were preferred, arteries were frequently used due to graft shortage. Although multivariable analysis did not show significant results, artery was a significant risk factor compared to vein graft in the univariable analysis of V5 reconstruction (HR, 4.667; 95% CI, 1.219–17.87; P = 0.025). Cryopreserved arteries are generally narrower than veins and the atherosclerosis of the intima is frequently observed. These features can induce venous stasis, which can lead to thrombosis.
The limitation of this study is that the study is designed as a retrospective study. However, the volumetric data achieved from patient CT was acquired before transplantation and the data were prospectively accumulated in the database. Since the program was started in 2019, we still do not have sufficient number of patients to perform a large-volume study. However, we managed to discriminate key factors related to the outcome of interest. Although the volumetric data acquisition failed to prove its clinical usefulness in graft survival and patient survival, it must be due to the high quality of clinical practice performed before the program. Still, the objective data guide the surgical team for better decision-making and allowed us to perform such a study to understand the volumetric information and the surgical outcome. Another limitation is the possible discrepancy between the preoperatively assessed volume and the reconstructed volume in cases where venous branches are multiple. The usual venous reconstruction of middle hepatic venous territory is up to 2 openings. In cases where 3 or more minor branches constitute the outflow, there may be some loss in outflow reconstruction. These volume data can be inaccurate since we only divided the data into V5 territory and V8 territory.
Before the introduction of the program, the surgical team decided to ligate or reconstruct the hepatic veins based on findings that indirectly represent the volume of the territory, which are vein opening length, flow of outflow perfusion during back-table procedure, or length of the intrahepatic venous branches. However, the volumetric data calculated directly gives the information on the importance of the need for outflow reconstruction. Due to the introduction of this program, we were able to perform this study and present objective data for estimating the risk of outflow occlusion. Of course, we did not show improvement in outflow patency. Nevertheless, the data presented by this study showed which cases can be vulnerable to outflow occlusion, and we consider that these cases can be candidates for preparing good quality cryopreserved vessels. There may be cases where liver graft in marginal and middle hepatic vein territory is proportionately large but less than 300 cm3 and V5 territory less than 150 cm3. The patency of the reconstructed outflow may be relatively important due to the high proportion, while the risk of occlusion can be increased due to small volume. We suggest that surgeons should take special consideration for these cases.
In conclusion, hepatic venous territory mapping allowed the surgical team to perform clinical practice based on objective data. Inferior hepatic vein and middle hepatic vein reconstruction is vital for the success of LDLT using right hemi-liver. Volumetric data achieved preoperatively could add beneficial information for the clinical practice of the transplantation team.
Go to :







 PDF
PDF Citation
Citation Print
Print









 XML Download
XML Download