Abstract
Purpose
Significant improvements have been made in the surgical treatment of rectal cancer with a higher sphincter-saving rate without compromising oncologic results. There have been studies about the quality of life of rectal cancer patients after surgery. However, no study has reported the long-term annual incidence of depression after rectal cancer surgery according to stoma status. The objective of this study was to determine the annual incidence of depression after rectal cancer surgery and the factors affecting it, especially the prevalence of depression according to the presence or duration of a stoma.
Methods
Using the Korea National Health Insurance Service database, patients who underwent radical surgery for rectal cancer from 2002 to 2019 were searched. We analyzed the incidence and risk factors of depression in patients who underwent radical surgery for rectal cancer according to stoma status.
Results
Annual incidence of depression in rectal cancer patients was decreasing annually for 15 years after surgery. There was no statistically significant difference in the incidence of depression according to the stoma status. However, the diagnosis of depression within 1 year after surgery was statistically significantly increased in the permanent stoma group.
Conclusion
There was no difference in the overall incidence of depressive disorders among patients with rectal cancer based on their stoma status. However, a permanent stoma seems to increase the incidence in the first year after surgery. Education and intensive assessments of depressive disorders in patients with permanent stoma within 1 year after surgery are needed, particularly for female patients who are under 50 years old.
Colorectal cancer is one of the most common cancers in the world. In Korea, colorectal cancer is the 3rd most newly diagnosed cancer (44.5 patients per 100,000 people in 2018) [1]. Significant improvements have been made in surgical techniques and oncologic results for the treatment of rectal cancer in the past 20 years. With the introduction of total mesorectal excision and the spread of preoperative chemoradiotherapy, local recurrence rate has significantly improved [2]. In addition, the sphincter-saving rate has been improved due to preoperative radiotherapy and technical advances in surgery for low rectal cancer such as intersphincteric resection [3]. However, rectal cancer itself and the resulting surgery may bring many quality-of-life (QOL) limitations. The resulting depressive mood may persist even after cure [4].
It has been reported that inflammation, chronic stress, cancer progression, and chemotherapy can also affect postoperative depression in rectal cancer patients [5]. Factors affecting QOL in rectal cancer patients also include low anterior resection syndrome (LARS) and the presence of stoma. Stoma patients may experience a significant number of technical, emotional, social, marital, and sexual difficulties [6]. Many studies have reported negative effects on body images, leading to increased anxiety and depression [78]. Not only stoma after sphincter-sacrificing rectal cancer surgery, but LARS may deteriorate the QOL after a sphincter-saving rectal cancer surgery [9]. There has been controversy over which is superior in QOL, but there may have been the unproven conviction that stoma more adversely affects QOL and that sphincter-saving surgery has superiority in all cases [1011].
Although numerous studies have analyzed the effect of stoma on QOL, few studies have reported effects of stoma in rectal cancer patients. Some studies have shown that permanent stoma has a negative effect on QOL [121314]. However, some studies on QOL of rectal cancer patients have suggested that the stoma itself has comparable effect on the QOL to patients without stoma after a rectal cancer surgery [11]. Rather than feeling just depressed due to stoma, finding out how many patients are actually diagnosed with depression can help predict significant clinical consequences of depression. Although many studies have used surveys related to the QOL and depression of rectal cancer patients, few studies on the actual morbidity of depression have been reported. In particular, since there was no study on long-term annual incidence of depression after surgery in rectal cancer patients, the objective of this study was to investigate annual incidence of depression after rectal cancer surgery and factors affecting it, especially the prevalence of depression according to the presence or duration of a stoma.
The study protocol was approved by the Institutional Review Board of Korea University College of Medicine (No. KUIRB-2021-0357-01). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature.
We analyzed the incidence of depression of rectal cancer patients using the Korea National Health Insurance Service (KNHIS) database. The KNHIS database contains patient demographics, International Classification of Disease (ICD)-based diagnostic codes, surgical and interventional codes, and prescription codes [15]. Personally identifiable information was deleted before analysis under the privacy protection law in Korea.
Using data registered in the KNHIS database, patients diagnosed with rectal cancer from 2002 to 2019 who underwent surgical treatment were searched using the ICD-10 system. Among patients diagnosed with C20 code of ICD-10, patients who underwent surgery related to cancer were extracted. Depressive disorder was searched for patients diagnosed with depressive disorder (F32) and recurrent depressive disorder (F33) in ICD code system. Patients with stomas were searched for stoma-related surgical codes using surgical codes in the KNHIS database. In case of surgery involving stoma creation, patients were classified as having stoma. Stoma reversal-related surgery (stoma take-down or reversal of Hartmann operation) was defined as termination of stoma. Those who had stoma reversal-related surgery were defined as temporary stoma. Those who had no stoma reversal-related surgery and who had abdominoperineal resection (APR) were defined as permanent stoma. Socioeconomic level was classified based on health insurance payment standards. Analysis was divided into the 20th deciles at 5% intervals from medical benefits.
Data were analyzed using other variables as covariates, including age, sex, radiotherapy (preoperative, postoperative, non), number of stoma-making surgeries (never, 1 time, 2 times, more than 3 times), socioeconomic status (Medicaid, 1–5, 6–10, 11–15, 16–20 quartiles), previous history of depression, and the presence or absence of the disease included in the Charlson comorbidity index (CCI) in each group.
To compare the demographic characteristics of individuals with and without stoma, we used the standardized difference between the 2 groups for both continuous and categorical variables. The absolute standardized difference of 0.10 or more indicates that covariates are imbalanced between groups. Follow-up started on the index date and was censored on the date of outcome occurrence, death, or last follow-up. The person-time at risk started on the index date and ended either at the time of depression diagnosis, at death, or at the end of the study period in 2019. We used the Kaplan-Meier method to estimate the cumulative incidences of depression. The incidences of depression with 95% confidence intervals (CI) were measured as the number of cases per 1,000 person-years. We measured incidence rate ratios (IRR) of depression in each group by using patients without a stoma as reference. The log-rank test was performed to examine differences in the risk for depression in the cohort. The IRR was used to examine the consistency over time.
The Cox proportional hazards models were used to compute the hazard ratios (HRs) accompanying 95% CIs after adjustment for confounders that are potentially associated with depression incidence, including age, sex, radiotherapy (preoperative, postoperative, non), number of stoma-making surgeries (never, 1 time, two times, more than 3 times), socioeconomic status (Medicaid, 1–5, 6–10, 11–15, 16–20 quartiles), previous history of depression and the CCI. We used Schoenfeld’s global test to test the proportional hazards assumption in the Cox proportional hazards model. We also conducted Cox regression analyses to examine differences in stoma type-depression associations according to age (<50 and ≥50 years) and survival period (<5 and ≥5 years). To correct bias due to cancer staging, sensitivity analyses were conducted by dividing the presence or absence of radiation treatment and the prior diagnosis of depression.
In order to compare the occurrence of depression in the general population, a matched comparator group was established. The comparator group without colorectal cancer comprised subjects without a diagnosis of colorectal cancer matched by age, sex, and socioeconomic level in a 1:4 ratio in each year during the enrollment period (2002–2019). The index date for the patients with rectal cancer was defined as the first diagnosis of rectal cancer during the study period. For the comparator group, the index date was the mean of the index dates for the matched rectal cancer patients in the matching year. A stratified Cox proportional hazards regression model considering the matched study design was used to calculate the adjusted HR and corresponding 95% CI for the relationship between rectal cancer with/without stoma and subsequent risk of depression. We considered a P-value of <0.05 as significant and all statistical tests were 2-sided. All of these analyses were conducted using STATA statistical software (ver. 15.0, StataCorp).
During the study period, 79,518 patients had surgical treatment for rectal cancer. Among them, 21,014 patients were excluded based on the exclusion criteria including 1,848 patients who were diagnosed with depression within 1 year before surgery (Supplementary Fig. 1). The mean age of these patients was 63.26 years (standard deviation [SD], 11.31). There were 37,230 males (63.64%). Among all patients, 33,778 (57.74%) had never had a stoma, 19,117 (32.68%) had a temporary stoma, and 5,609 (9.59%) had a permanent stoma. At the time of diagnosis, the most common age was those in their 60s (31.02%), followed by those in their 70s (25.87%) and 50s (24.82%).
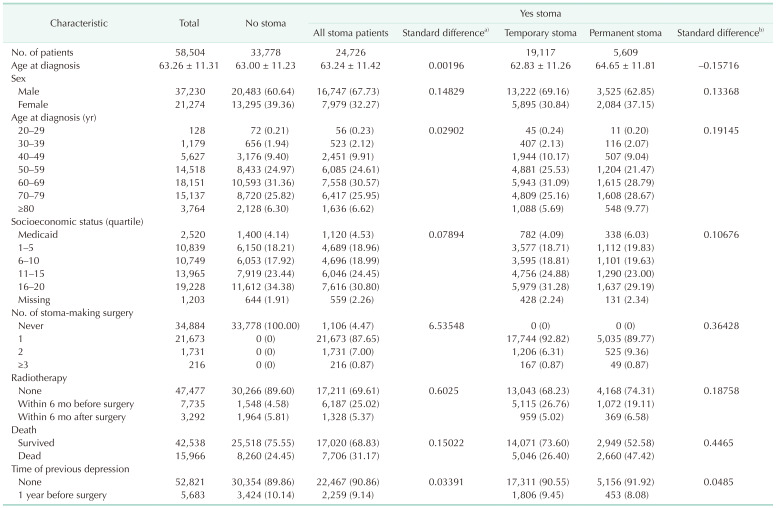
Table 1 provides a comparison between all stoma patients and patients without a stoma, as well as between patients with temporary and permanent stomas within the stoma patient group. The number of cases of having a stoma was higher in males than in females (standard difference, 0.15). Patients who received radiation therapy were more likely to have stomas than those who did not (standard difference, 0.60). It was also found that stoma was more common in patients who died during the study period (standard difference, 0.15). There was no difference in the previous depression diagnosis 1 year prior to surgery between the patients with stoma and without stoma (standard difference, 0.03). Compared to the temporary stoma group, the permanent stoma group showed higher proportion of females patients, the patients with old age at diagnosis, the patients who underwent 2 or more surgeries to make stoma and the patients who did not receive radiation therapy (Table 1).
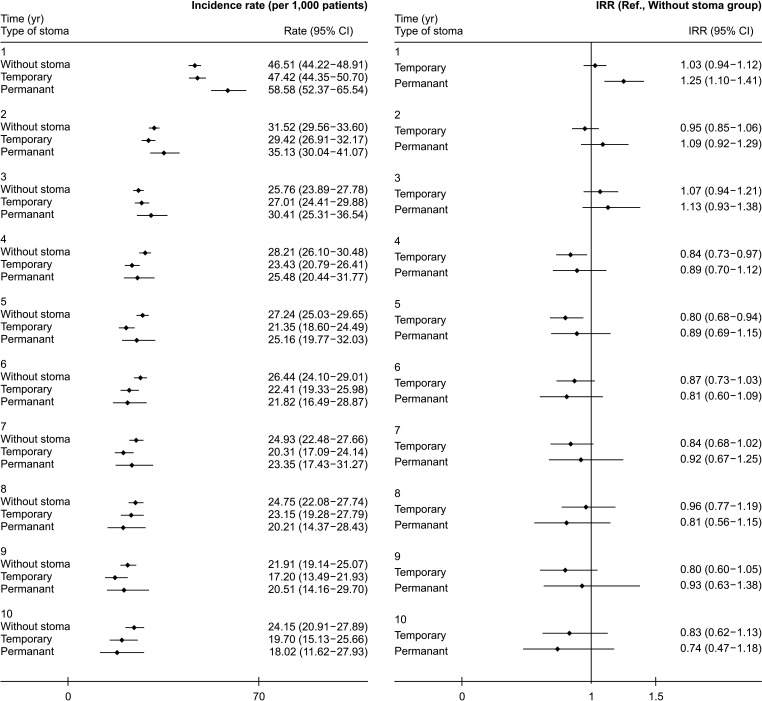
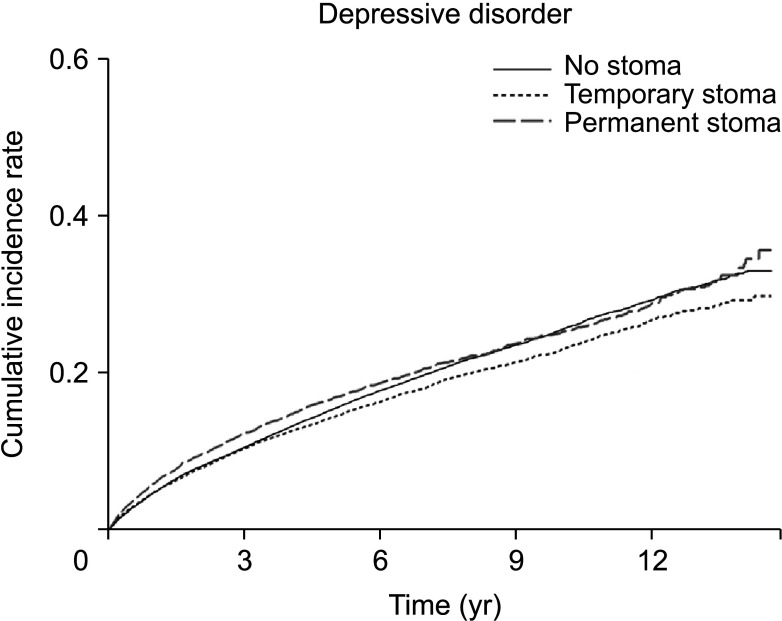
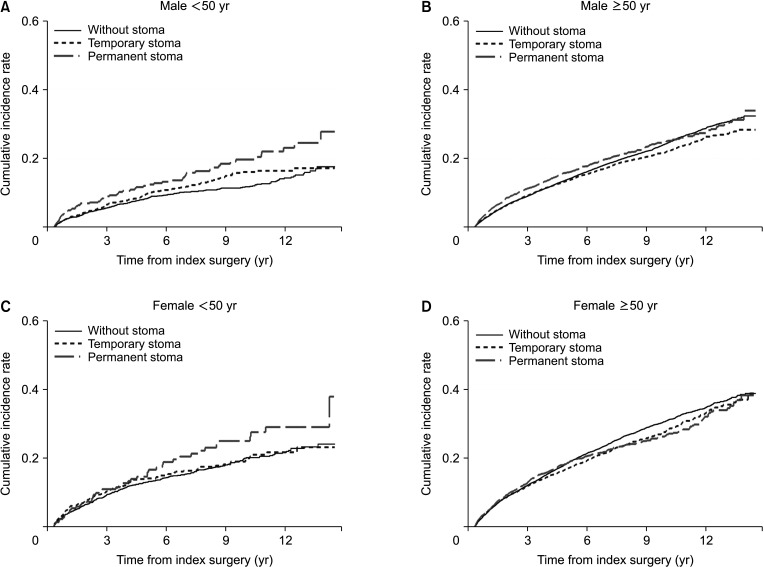
Cumulative incidence throughout the study period of depression among rectal cancer patients was 18.98% in patients without stoma, 15.64% in those with a temporary stoma, and 18.42% in those with a permanent stoma. We investigated the incidence of depression over time after surgery during the study period. The incidence of depression per 1,000 person-year at 1, 3, and 5 years was 47.53, 25.76, and 27.24 in the no stoma group, 47.42, 27.01, and 21.35 in the temporary stoma group, and 58.58, 30.41, and 25.16 in the permanent stoma group (Supplementary Table 1). At 1 year after surgery, the number of patients who were diagnosed with depressive disorder in the permanent stoma group was 58.58 per 1,000, which was significantly larger than that in the no stoma group or the temporary stoma group (IRR, 1.25; 95% CI, 1.10–1.41). However, it was not significantly different between 2 years and 15 years after surgery (Figs. 1, 2; Supplementary Table 1).
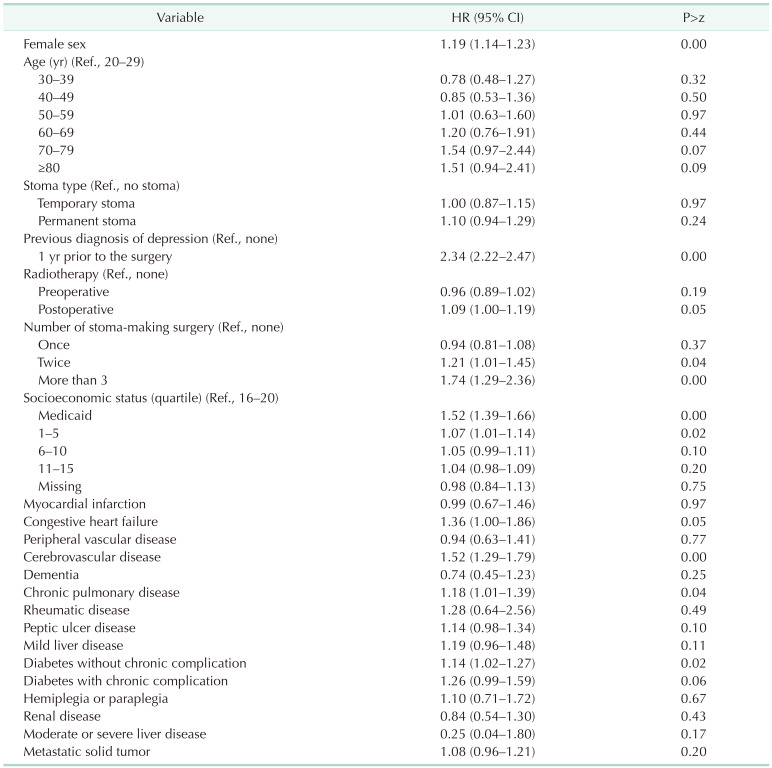
In all rectal cancer patients, more females than males were diagnosed with depression (HR, 1.19; 95% CI, 1.14–1.23). The incidence of depression showed no difference according to age. However, it was higher in males in their 70s and 80s than that in males in their 20s (HR, 2.76 for 70s and 2.99 for 80s) (Supplementary Table 2). The incidence of depression increased after rectal cancer surgery if there was a history of depression more than 1 year before surgery (HR, 2.34; 95% CI, 2.22–2.47). It increased when stoma-making surgeries were performed 2 times or more (HR, 1.21; 95% CI, 1.01–1.45 for 2 times and HR, 1.74; 95% CI, 1.29–2.36 for more than 3 times). The lower the socioeconomic level, the higher the incidence of depression. In addition, the incidence of depression was higher in patients with cerebrovascular disease, diabetes mellitus without chronic complication, and chronic pulmonary disease (Table 2).
To correct bias that can happen due to different cancer staging, subgroup analysis was performed by dividing the presence or absence of radiation treatment perioperatively and according to survival of more than 5 years. There were no significant differences in overall results (Supplementary Tables 3, 4). Although the wash-out period for depression was set to be 1 year, depression before 1 year might influence depression after surgery. Therefore, another analysis was conducted by dividing subjects by the presence or absence of depression more than 1 year before surgery. Similarly, there were no significant differences in overall results (Supplementary Table 5).
There was no difference in the incidence of depression according to the presence or absence of a stoma or whether it is temporary or permanent (Table 2). This trend showed similar results according to sex. However, when analyzed by age, it was confirmed that depression was significantly increased in those under 50 years when they had a permanent stoma (HR, 1.85; 95% CI, 1.17–2.92) (Fig. 3, Supplementary Table 2).
This is the first study that provides long-term cumulative incidence of depressive disorder in rectal cancer according to stoma status using nationwide population-based data. It has some important strengths. The first one is the reliability of the data that this study roots on. National Health Insurance covers almost 98% of the total population (approximately 50 million as of 2014) in Korea [16]. In Korea’s national medical insurance system, when cancer is diagnosed, the deductible is only 5% of copayments. Therefore, data are highly reliable because of the strict review of cancer diagnosis and codes of cancer surgery and related prescriptions. Secondly, although there were studies comparing QOL at a certain point in time previously, this is the first study on long-term annual incidence of depression in rectal cancer patients.
In the United States, the prevalence of major depressive disorder in 2005 was 6.8%. It rose to 7.1% in 2018 [17]. The prevalence of depression in Korea in 2013 was 5.5%, which was similar to that in other high-income countries [1819]. In a review of global prevalence of depression, the annual incidence of depressive disorder was found to be 2.4% [20]. When looking at results of our study, in the case of permanent stoma, it was found that the incidence of depressive disorder was about twice that of the global data as it was 5.9% in the first year after surgery. However, it was confirmed that the annual incidence of depressive disorder decreased to the average global incidence from the 4th year (Supplementary Table 1). Risk factors for depression include females, the elderly, and high or low socioeconomic status [19]. Similarly, in our study, depression was more common in females and older adults. However, the incidence of depression was higher in the low group in terms of socioeconomic level. This might be due to a relatively high probability of having a permanent stoma if the socioeconomic level is low (Table 1).
Various factors can affect postoperative depression in rectal cancer patients. In general, there are psychological and social factors in the pathogenesis of various depression disorders in cancer patients. Inflammation, chronic stress, cancer progression, and chemotherapy can also affect depression [5]. Factors affecting QOL related to rectal cancer also include LARS and the presence of stoma. Stoma is generally made in 2 cases: (1) when the anal function is lost, such as abdominoperineal excision or severe fecal incontinence; and (2) when there is diversion. If there is a high possibility of leakage after anastomosis after bowel resection, the end of the proximal intestine is taken out after anastomosis to improve the clinical course even if leakage occurs. Particularly after rectal cancer surgery, diverting stoma can be made because the anastomotic leakage rate is higher in rectal surgery than in the colon. Risk factors such as preoperative radiation, males, narrow pelvis, and low tumor location are associated with high leakage rate. Although diverting stoma does not reduce the leakage rate itself, stoma is made because it makes the clinical course better after leakage. Recently, concurrent chemoradiotherapy (CCRT) has increased since preoperative CCRT can decrease local recurrence rate in some rectal cancers, so diversion has increased accordingly.
Thorpe et al. [21] have studied physical and psychological adaptation processes after stoma surgery by interviewing patients at 3, 9, and 15 months after stoma surgery. They found that having a stoma can cause a collapse of the unconscious relationship between the self and the body that existed before the operation. They also found that having a stoma caused patients to develop negative emotions and distance themselves from their physical changes in order to regain a ‘normal’ self. However, they said that these changes showed an adaptation at the time of about 1 year after the operation. Another study has reported that there is no difference in scores among patients who are able to be assessed with the adjustment scoring at 4 months, 1 year, and 4 years after stoma surgery. It also reported that there was no difference in scoring regardless of whether the cause of the stoma was a benign disease or a malignant disease [22]. Researchers have also reported that a depressive mood can persist even for 5 years after surgery. Survivors after a rectal cancer surgery have an overall normal QOL, although their depression tends to increase more than that in the general population and appears to persist 5 years after surgery. This is thought to be the fear of recurrence after surgery and the stress of continuing diagnostic tests [23]. However, in this study, there was no difference in the diagnosis of depression depending on the presence or duration of a stoma.
Complications such as LARS might be associated with increased mood disorders in rectal cancer patients [24]. In a longitudinal cohort study of Scandinavian patients, about 63% and 56% of patients who recovered bowel continuity after rectal cancer surgery developed major LARS at 1 year and 2 years after surgery, respectively [25]. In a study comparing the QOL between APR patients and sphincter-saving surgery patients 1 year after rectal cancer surgery, there was no difference in overall QOL. However, a characteristic QOL decrease in APR patients was observed, although data after 1 year were not presented [26]. Walming et al. [13] have reported that in patients with resectable rectal cancer, the QOL immediately after surgery was lowered, but recovered to the level of the general population after 1 and 2 years, in line with results of our study. Although follow-up was carried out only for 2 years after surgery in this study, and no analysis was made according to the presence or absence of stoma, they reported that poor bowel or stoma function is a risk factor for QOL deterioration. In our study, after the first year of adaptation period, the incidence of depression decreased regardless of the presence or type of stoma. It can be inferred that the depression is greater in the case of permanent stoma during the first year.
Our study found that the presence of a stoma did not have an impact on the overall incidence of depression. From 2 years after surgery, the same annual incidence of depression was observed regardless of the presence or absence of a stoma. However, within the first year following surgery, the permanent stoma group had a higher incidence of depression diagnosis compared to other groups. In the case of a permanent stoma, an increase in overall depression was observed in young females under 50 years of age. Therefore, it is necessary to educate about stoma before surgery and take active measures necessary for adaptation after surgery in cases predicted to have a permanent stoma or in young females. Patient education on stoma before and after rectal cancer surgery is an essential element of patient management for functional and psychological recovery of stoma patients. And we can also adopt preoperative psychological education, cognitive-behavioral therapy, and psychological support for stoma patients [727].
Studies targeting long-term survivors of colorectal cancer have reported that long-term factors affecting QOL even after full recovery from colorectal cancer are the presence of stoma, changes in bowel function, and cancer-related distress [23]. In a comparative analysis with the general population in this study, the annual incidence of depression was similar in the temporary stoma group and no stoma group from 5 years after surgery. In the permanent stoma group, all of them had a significantly higher depression diagnosis rate compared to the general population within three to 5 years after surgery. However, after 5 years, depression incidence in rectal cancer patients was similar to the general population regardless of the stoma status. This is probably because they became more and more free from cancer recurrences. Although there may be memories of cancer experiences, complications due to surgery, or changes in bowel function, it is interesting that after three to 5 years, regardless of the type of stoma, all of them became to have similar incidence rate of depression to the general population. Another interesting point is that the permanent stoma group had depression more often than the general population in the first year after surgery. However, they recovered to the general population level of depression incidence more quickly (3 years after surgery) than the temporary or no stoma group (Supplementary Fig. 2). These can be explained by reframing/response shift. It is assumed that cancer survivors can establish new meanings of the QOL concept or change the composition of the QOL dimension [2829].
After rectal cancer surgery, LARS might occur due to nerve injury and other factors. However, the effect of LARS on QOL reduction was unknown in this study. Many patients who had stoma after rectal cancer surgery might have had LARS after stoma was taken down. LARS is associated with low QOL. Some patients even rebuild stoma due to LARS. Therefore, the difference in depression occurrence in the permanent stoma group might have been offset to some extent because patients in the no stoma group and the temporary stoma group were affected by LARS. Interestingly, there was no difference in the incidence of depression according to the presence or absence of a permanent stoma. Difficulty due to bowel problems after rectal cancer surgery might have a counteracting effect or is related to reframing/response shift.
This study has some limitations. First, there was no correction for cancer staging. Patients with higher stages are more likely to have CCRT and stoma. In addition, the survival in such patients is likely to be lower. The higher the stage in the stoma group, the higher the recurrence and the more mood disorders. However, to correct for the effect of stage, we performed additional analysis according to the presence or absence of radiation treatment and survival of more than 5 years. Similarly, there was no difference in the incidence of depression. However, we still could not conduct an analysis including control for whether or not chemotherapy was given, and we could not control some situations such as Hartmann surgery in an emergency setting. Secondly, it was not possible to analyze many factors that could be related to the onset of depression, such as various events that could cause stress and emotional support from acquaintances. Thirdly, we did not control for other psychiatric conditions that could affect the diagnosis of depression. Lastly, similar to other psychiatric disorders, these cohort studies based on large samples may underestimate the true prevalence in the general population.
Despite these limitations, it is believed that this study using a large-scale database for the entire population of Korea provides important information about the diagnostic pattern and risk of depression in rectal cancer patients.
In patients with rectal cancer, stoma was not related to the diagnosis of depression. However, the presence of postoperative permanent stoma seems to increase the incidence of depressive disorders in the first year after surgery. Also, permanent stoma increased the diagnosis of depression in young patients under 50 years of age. Additionally, depression was also found to be associated with recurrent stoma-making surgery. Thus, is it important to provide education before surgery and conduct intensive assessment of depressive disorders within 1 year after surgery.
Notes
References
1. Khil H, Kim SM, Hong S, Gil HM, Cheon E, Lee DH, et al. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci Rep. 2021; 11:2413. PMID: 33510236.
2. Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997; 336:980–987. PMID: 9091798.
3. Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, et al. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009; 249:236–242. PMID: 19212176.
4. Chongpison Y, Hornbrook MC, Harris RB, Herrinton LJ, Gerald JK, Grant M, et al. Self-reported depression and perceived financial burden among long-term rectal cancer survivors. Psychooncology. 2016; 25:1350–1356. PMID: 26365584.
5. Smith HR. Depression in cancer patients: pathogenesis, implications and treatment (Review). Oncol Lett. 2015; 9:1509–1514. PMID: 25788991.
6. Follick MJ, Smith TW, Turk DC. Psychosocial adjustment following ostomy. Health Psychol. 1984; 3:505–517. PMID: 6536500.
7. Clark M, Chur-Hansen A, Mikocka-Walus A. Systematic review with meta-analysis: current and emerging models of preoperative psychological preparation for individuals undergoing stoma surgery. J Psychosom Res. 2023; 168:111211. PMID: 36898315.
8. Hong KS, Oh BY, Kim EJ, Chung SS, Kim KH, Lee RA. Psychological attitude to self-appraisal of stoma patients: prospective observation of stoma duration effect to self-appraisal. Ann Surg Treat Res. 2014; 86:152–160. PMID: 24761424.
9. Emmertsen KJ, Laurberg S. Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg. 2013; 100:1377–1387. PMID: 23939851.
10. Kang SB, Cho JR, Jeong SY, Oh JH, Ahn S, Choi S, et al. Quality of life after sphincter preservation surgery or abdominoperineal resection for low rectal cancer (ASPIRE): a long-term prospective, multicentre, cohort study. Lancet Reg Health West Pac. 2020; 6:100087. PMID: 34327411.
11. Pachler J, Wille-Jørgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2012; 12:CD004323. PMID: 23235607.
12. Näsvall P, Dahlstrand U, Löwenmark T, Rutegård J, Gunnarsson U, Strigård K. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res. 2017; 26:55–64. PMID: 27444778.
13. Walming S, Asplund D, Bock D, Gonzalez E, Rosenberg J, Smedh K, et al. Quality of life in patients with resectable rectal cancer during the first 24 months following diagnosis. Colorectal Dis. 2020; 22:2028–2037. PMID: 32871612.
14. Song L, Han X, Zhang J, Tang L. Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer. Psychooncology. 2020; 29:796–802. PMID: 32043668.
15. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017; 46:799–800. PMID: 27794523.
16. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–728. PMID: 28378543.
17. Proudman D, Greenberg P, Nellesen D. The growing burden of major depressive disorders (MDD): implications for researchers and policy makers. Pharmacoeconomics. 2021; 39:619–625. PMID: 34013439.
18. Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011; 9:90. PMID: 21791035.
19. Kim GE, Jo MW, Shin YW. Increased prevalence of depression in South Korea from 2002 to 2013. Sci Rep. 2020; 10:16979. PMID: 33046758.
20. Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013; 43:471–481. PMID: 22831756.
21. Thorpe G, Arthur A, McArthur M. Adjusting to bodily change following stoma formation: a phenomenological study. Disabil Rehabil. 2016; 38:1791–1802. PMID: 26930444.
22. Bekkers MJ, van Knippenberg FC, van Dulmen AM, van den Borne HW, van Berge Henegouwen GP. Survival and psychosocial adjustment to stoma surgery and nonstoma bowel resection: a 4-year follow-up. J Psychosom Res. 1997; 42:235–244. PMID: 9130180.
23. Jansen L, Koch L, Brenner H, Arndt V. Quality of life among long-term (≥5 years) colorectal cancer survivors: systematic review. Eur J Cancer. 2010; 46:2879–2888. PMID: 20605090.
24. Sprangers MA, Taal BG, Aaronson NK, te Velde A. Quality of life in colorectal cancer. Stoma vs. nonstoma patients. Dis Colon Rectum. 1995; 38:361–369. PMID: 7720441.
25. Sandberg S, Asplund D, Bisgaard T, Bock D, González E, Karlsson L, et al. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: a longitudinal follow-up within the QoLiRECT study. Colorectal Dis. 2020; 22:1367–1378. PMID: 32346917.
26. Russell MM, Ganz PA, Lopa S, Yothers G, Ko CY, Arora A, et al. Comparative effectiveness of sphincter-sparing surgery versus abdominoperineal resection in rectal cancer: patient-reported outcomes in National Surgical Adjuvant Breast and Bowel Project randomized trial R-04. Ann Surg. 2015; 261:144–148. PMID: 24670844.
27. Fioravanti M, Di Cesare F, Ramelli L, La Torre F, Nicastro A, Messinetti S, et al. Pre-surgery information and psychological adjustment to enterostomy. Ital J Surg Sci. 1988; 18:55–61. PMID: 3372216.
28. Bernhard J, Lowy A, Mathys N, Herrmann R, Hürny C. Health related quality of life: a changing construct? Qual Life Res. 2004; 13:1187–1197. PMID: 15473497.
29. Bernhard J, Hürny C, Maibach R, Herrmann R, Laffer U. Swiss Group for Clinical Cancer Research (SAKK). Quality of life as subjective experience: reframing of perception in patients with colon cancer undergoing radical resection with or without adjuvant chemotherapy. Ann Oncol. 1999; 10:775–782. PMID: 10470423.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–5 and Supplementary Figs. 1, 2 can be found via https://doi.org/10.4174/astr.2023.104.6.303.
Supplementary Fig. 2
Risk of depression of no stoma/temporary stoma group and permanent stoma group comparing to the general population along time after index operation. HR, hazard ratio; CI, confidence interval.
Supplementary Table 1
Depression incidence by year and age according to stoma status
Supplementary Table 2
Risk factors of depression according to sex and age in rectal cancer patients
Supplementary Table 3
Risk factors for depression in rectal cancer patients with and without radiation therapy before and after surgery
Supplementary Table 4
Risk factors of depression according to survival period in rectal cancer patients
Supplementary Table 5
Risk factors of depression when limited to patients with no previous history of depression




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download