Abstract
Purpose
Venoactive drugs are widely used to improve the symptoms and signs of chronic venous disease. This study aimed to analyze the rate of adverse events after venoactive drug prescription and subsequent compliance and switching rates.
Methods
Using the National Health Insurance Service database, individuals with at least one chronic venous disease code between January 2009 and December 2019 were identified, and 30% (2,216,780 individuals) of these were sampled. Finally, 1,551,212 patients were included, and we analyzed adverse events, compliance, and switching rates with 8 venoactive drugs, including Vitis vinifera extract, naftazone, micronized purified flavonoid fraction, Vitis vinifera leaf extract, diosmin, diobsilate calcium, bilberry fruit dried extract, and sulodexide.
Results
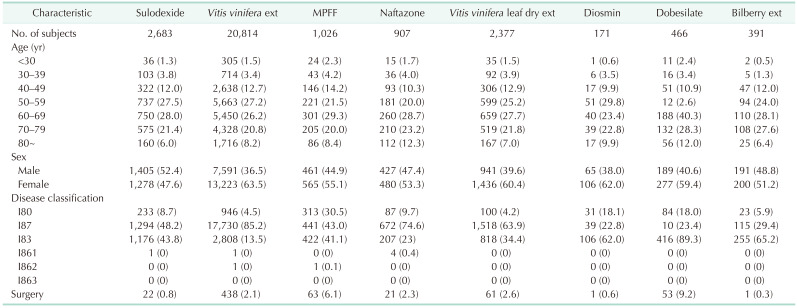
The most commonly prescribed venoactive drug was Vitis vinifera extract (72.2%), followed by sulodexide (9.3%), and Vitis vinifera leaf dry extract (8.2%). Adverse event rates were significantly lower in the naftazone and diosmin groups (P = 0.001 and P = 0.002, respectively) and significantly higher in the Vitis vinifera leaf dry extract group (P = 0.009). Drug adherence to sulodexide was the highest throughout the study period, followed by billberry extract and dobesilate (all P < 0.001). For most drugs, the drug switching rate was low (<5.0%).
Chronic venous disease (CVD) is defined as an abnormally functioning venous system due to venous valvular incompetence with or without associated venous outflow obstruction; this may affect the superficial venous system, deep venous system, or both. The goal of CVD treatment is to decompress the sources of increased venous pressure, and a wide range of treatment options are currently available, both conservative and invasive [1].
Pharmacological treatment with venoactive drugs (VADs) can improve venous tone and contractility, reduce edema and inflammation, and improve microcirculation [2]. The main modes of action of VADs are decreasing capillary permeability and reducing release of inflammatory mediators [1]. Compared with placebo, VADs may have beneficial effects on objective measures of leg edema and on some signs and symptoms related to CVD, such as pain, cramps, restless legs, sensation of swelling, paresthesia, and trophic disorders [3]. VADs are associated with adverse effects, and the selection of VAD is usually based on drug-related adverse events or efficacy [4].
Currently, several VADs are frequently prescribed in Korea, but there have been no reports on compliance or tolerability for each VAD. This study aimed to analyze the rate of adverse events after VAD prescriptions, as well as compliance and switching rates.
This retrospective cohort study used National Health Insurance (NHI) claims data from the anonymized public data provided by the National Health Insurance Service (NHIS). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (KHNMC 2019-10-005).
This study used the NHIS data from patients with CVD between January 2009 and December 2019. Since the introduction of the NHIS in 1989, the Korean government has collected patient medical records and information regarding medical resource utilization. Since all citizens are obligated to join, the NHI program includes more than 98% of citizens. The Health Insurance Review and Assessment Service has built a national database using medical expense claims data submitted by medical institutions to provide real-world data. NHIS data are recorded as claims for medical service items provided to patients, including diagnosis, treatment, and prescription information from anonymized personal identification numbers.
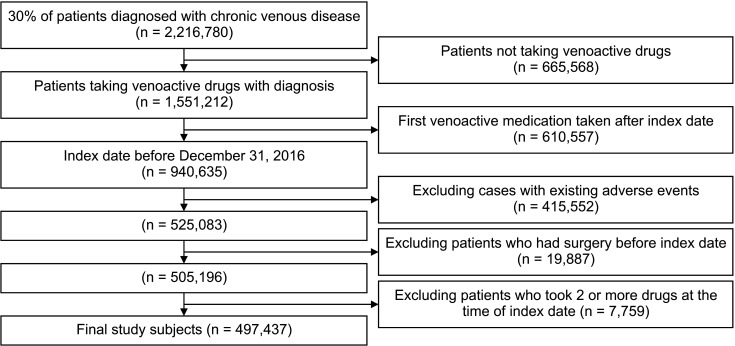
A flow diagram is shown in Fig. 1. Among individuals with at least one CVD code (including subcodes ICD-10 I80, I83, I861, I862, I863, and I87) between January 2009 and December 2019, 30% (2,216,780 persons) were randomly sampled due to the data provision regulations of NHIS. Data from 1,551,212 patients who received a prescription for drugs to treat CVDs (Vitis vinifera extract, naftazone, micronized purified flavonoid fraction [MPFF], Vitis vinifera leaf extract, diosmin, dobesilate calcium, bilberry fruit dried extract, sulodexide) were selected for analysis. The date of the first VAD prescription was set as the index date. Patients with an index date of 2017 or later were excluded if they had a follow-up period of >2 years. Patients who underwent venous procedures before the index date and those who used combined VADs at the index date were excluded from the analysis to avoid difficulty in analyzing drug-related outcomes. Finally, 497,437 participants were included in the study. The analysis only included cases in which the sum of prescription days for each drug after the index date was at least 70% (129 days or more) within the first 6 months.
The primary outcomes were drug compliance and withdrawal. Drug adherence was analyzed for each case where the sum of the prescription days for the first medication was ≥80% within 3 months, 6 months, 1 year, and 2 years after the index date. The rates of drug withdrawal, termination, and combination with other diseases were also evaluated. The withdrawal period was defined as no record of drug use for CVD for >60 days. Cases in which 2 or more drugs were taken together during the study period were defined as drug combinations.
Adverse events were confirmed for each case where there was a code mentioned as a side effect of the drug in the Ministry of Food and Drug Safety approval documents (Supplementary Table 1). Adverse events were confirmed for cases that occurred within 3 months, 6 months, 1 year, and 2 years, and within the entire period until the first occurrence after the index date. The number of procedures or surgeries performed for varicose veins during the study period was also analyzed.
Demographic characteristics and outcome variables are presented as frequencies and percentages. Univariate logistic regression analysis was performed to analyze whether surgery or adverse events occurred depending on the types of drugs taken and whether drug compliance differs by the type of medication taken for the first time. The outcome variables are presented as odds ratios (ORs), 95% confidence intervals (CIs), and P-values. Two-sided significance tests were performed with the significance level set at 0.05. Patients were divided into 5 age groups with 10-year intervals: <30, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years. All analyses were performed using SAS software ver. 7.1 (SAS Institute).
The baseline characteristics of the study population are summarized in Table 1. The most commonly prescribed venotonic drug was Vitis vinifera extract (n = 20,814, 72.2%), followed by sulodexide (n = 2,683, 9.3%), and Vitis vinifera leaf dry extract (n = 2,377, 8.2%). The prescription rate was higher in women than in men (60.9% vs. 39.1%). Regarding age groups, the prescription rate was highest in those in their 60s, followed by those in their 50s and 70s (26.9%, 26.2%, and 21.2%, respectively). The numbers of patients who underwent surgery while taking the drug were highest in the dobesilate and MPFF groups (9.2% and 6.1%, respectively) and lowest in the bilberry extract group (0.3%).
Drug compliance was evaluated based on adherence and switching to other drugs (Figs. 2, 3). Drug adherence to sulodexide was the highest throughout the study period, followed by bilberry extract and dobesilate (all P < 0.001). Drug adherence was significantly lower for naftazone, diosmin, and MPFF (all P < 0.001). When compared to the rest of the drugs, adherence to Vitis vinifera extract was lower at 3 months but higher at 2 years, while that of Vitis vinifera leaf dry extract was higher at 3 months but lower at 2 years (all P < 0.05).
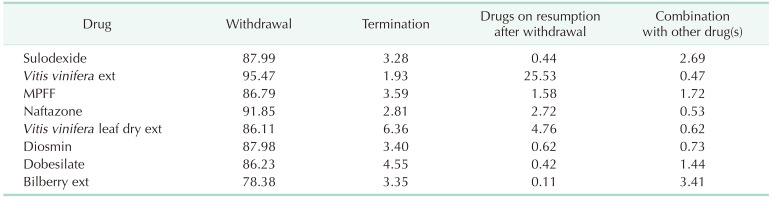
For most drugs, the drug switching rate was less than 5%. Vitis vinifera extract was most commonly used as a combination or switch drug, whereas bilberry extract was least frequently chosen as a switch drug. Table 2 summarizes drug withdrawal, termination, and combination rates. The drug withdrawal rate was lowest for bilberry extract (78.33%) and highest for Vitis vinifera extract (95.47%). Termination was highest in Vitis vinifera leaf dry extract (6.36%). Combination treatment with 2 or more drugs was observed in less than 5% of patients. Bilberry extract (3.41%) and sulodexide (2.69%) were most commonly used in combination treatments.
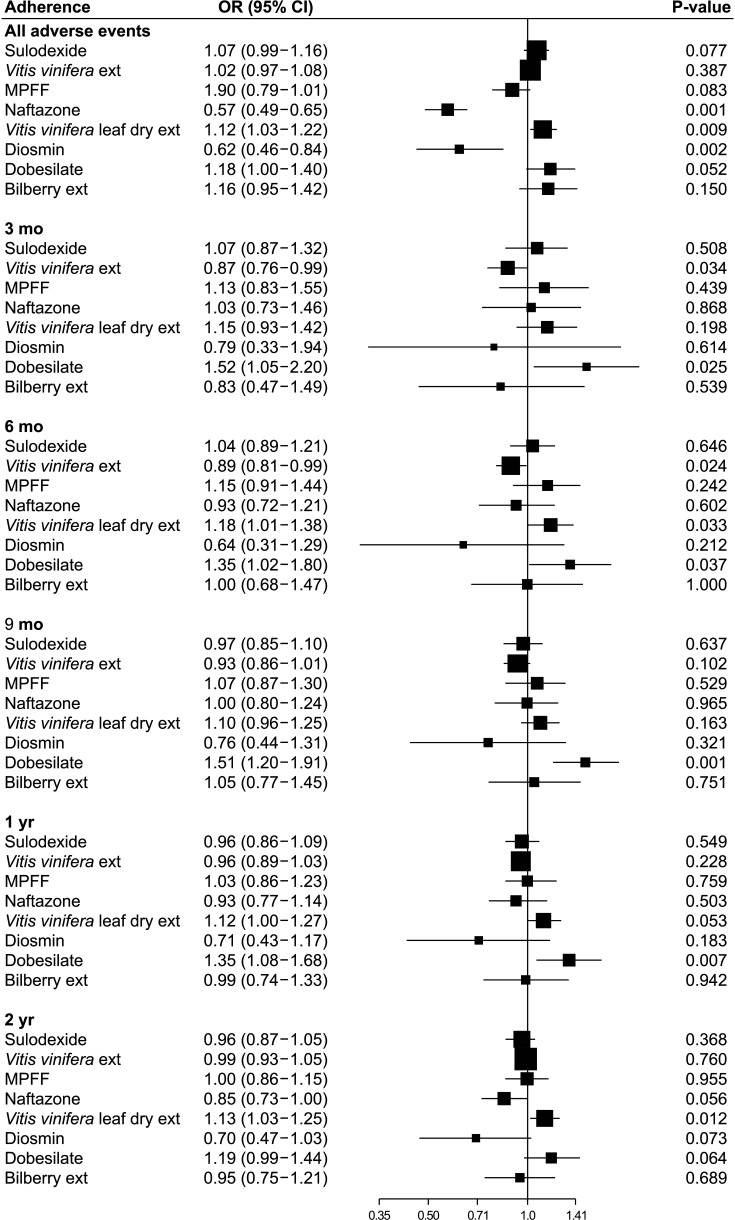
The risk of adverse events is depicted in Fig. 4. During the entire study period, adverse events were significantly lower in the naftazone and diosmin groups (P = 0.001 and P = 0.002, respectively), and significantly higher in the Vitis vinifera leaf dry extract group (P = 0.009). At 3 months, adverse events were lower in the Vitis vinifera extract group than in the other (OR, 0.87; 95% CI, 0.76–0.99; P = 0.034). Adverse events associated with dobesilate were significantly higher than those associated with other drugs at 3, 6, 9, and 12 months (P = 0.025, P = 0.037, P = 0.001, and P = 0.007, respectively). Interestingly, the adverse events of Vitis vinifera extract were significantly lower at 3 and 6 months (P = 0.034 and P = 0.024, respectively), while those of the Vitis vinifera leaf dry extract were not significantly different from those of the other drugs at 3 months (P = 0.198) and were significantly higher at 6 months and 2 years (P = 0.033 and P = 0.012).
CVD is a prevalent disease with a wide spectrum of clinical manifestations [5]. The Clinical Etiology Anatomy Pathophysiology (CEAP) classification of CVD is widely used to describe CVD stages based on clinical manifestations and other characteristics of the disease, and the choice of CVD treatment used to improve patients’ symptoms depends on the CEAP stage [6]. VADs are frequently prescribed to patients with CVD to alleviate symptoms of venous hypertension by increasing venous tone [78]. A recent systematic review suggested that VADs were effective in reducing edema, but were associated with a higher risk of adverse events than placebo [3]. In this study, we purposed to evaluate efficacy and safety directly by analyzing drug compliance and switching rates for individual VADs available in Korea. In our study, Vitis vinifera extract was the most commonly prescribed drug. An interesting finding of this study was that the risk of adverse events and rates of drug compliance did not match. The compliance was highest with sulodexide, Vitis vinifera leaf dry extract, and dobesilate, but the adverse events were highest in Vitis vinifera leaf dry extract and dobesilate. This finding is contrary to the belief that adverse drug events affect drug compliance. This result can be interpreted in several ways: first, the adverse events did not seem to be serious enough to reduce compliance. The most common adverse events of Vitis vinifera leaf dry extract and dobesilate were urticaria and gastrointestinal disturbances [910], but these drugs were relatively well tolerated and the adverse events were not serious [11]. Second, better compliance with these drugs might result from better symptom improvement that outweighs the risk due to adverse events. Regarding the efficacy of these drugs, while the recommendation level was weak with a moderate quality of evidence, decreases in limb volume and pain were reported [10121314]. Therefore, further studies are required to clarify the efficacy of these drugs. Third, the relatively high compliance might have resulted from the prescription by a physician. The switching rate was relatively low (>5%) for most of the drugs and similar for all VADs, and the patients tended to comply well with the physician’s initial prescriptions.
At present, the efficacy of VADs has been demonstrated by meta-analyses and comparative trials, and Ruscus + hesperidin methylchalcone (HMC) + vitamin C and MPFF have been shown to be effective, with high-quality evidence [1215]. Despite the high level of evidence, the Ruscus + HMC + vitamin C formulation is not yet widely used in Korea, and MPFF is not used as frequently as other drugs. More evidence on the efficacy and safety of the included drugs is needed for evidence-based decision-making, as most evidence for other VADs is weak [12].
A limitation of this study is the lack of information on patients, including CVD severity, symptoms, type of institution where they were treated, or severity of adverse events, which could potentially affect drug compliance and switching rates. Second, owing to the limited amount of data provided by the NHIS, only a 30% sample was provided for this study. Since it was extracted by a random algorithm of the NHIS, the sample should be representative, but the possibility of selection bias cannot be excluded. Despite these limitations, this study has strength in that it is the first to analyze VAD prescription status through national database analysis and compare adherence and adverse events for each drug.
References
1. De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren L, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022; 63:184–267. PMID: 35027279.
2. Dialsingh I, Mohammed SR, Medford RS, Budhoo E, White-Gittens I, Maharaj D. Conservative therapy significantly reduces patients’ chronic venous disease symptoms: a Caribbean insight into the VEIN Act Program. Phlebology. 2022; 37:651–661. PMID: 35848710.
3. Martinez-Zapata MJ, Vernooij RW, Simancas-Racines D, Uriona Tuma SM, Stein AT, Moreno Carriles RM, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2020; 11:CD003229. PMID: 33141449.
4. Cazaubon M, Benigni JP, Steinbruch M, Jabbour V, Gouhier-Kodas C. Is there a difference in the clinical efficacy of diosmin and micronized purified flavonoid fraction for the treatment of chronic venous disorders? Review of available evidence. Vasc Health Risk Manag. 2021; 17:591–600. PMID: 34556990.
5. Ortega MA, Fraile-Martínez O, García-Montero C, Álvarez-Mon MA, Chaowen C, Ruiz-Grande F, et al. Understanding chronic venous disease: a critical overview of its pathophysiology and medical management. J Clin Med. 2021; 10:3239. PMID: 34362022.
6. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018; 19:1669. PMID: 29874834.
7. Sheikh P, Lohsiriwat V, Shelygin Y. Micronized purified flavonoid fraction in hemorrhoid disease: a systematic review and meta-analysis. Adv Ther. 2020; 37:2792–2812. PMID: 32399811.
8. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011; 41:117–125. PMID: 21126890.
9. Allain H, Ramelet AA, Polard E, Bentué-Ferrer D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004; 27:649–660. PMID: 15230646.
10. Rabe E, Stücker M, Esperester A, Schäfer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency: results of a double-blind placebo-controlled study. Eur J Vasc Endovasc Surg. 2011; 41:540–547. PMID: 21239190.
11. Belcaro G. A clinical comparison of pycnogenol, antistax, and stocking in chronic venous insufficiency. Int J Angiol. 2015; 24:268–274. PMID: 26648668.
12. Nicolaides A, Kakkos S, Baekgaard N, Comerota A, de Maeseneer M, Eklof B, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Part I. Int Angiol. 2018; 37:181–254. PMID: 29871479.
13. Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS 195: a randomised, double-blind, placebo-controlled, crossover study. Drugs R D. 2004; 5:63–71. PMID: 15293865.
14. Ciapponi A, Laffaire E, Roqué M. Calcium dobesilate for chronic venous insufficiency: a systematic review. Angiology. 2004; 55:147–154. PMID: 15026869.
15. Senra Barros B, Kakkos SK, De Maeseneer M, Nicolaides AN. Chronic venous disease: from symptoms to microcirculation. Int Angiol. 2019; 38:211–218. PMID: 31112025.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2023.104.5.288.
Fig. 2
Drug adherence. Each drug with reference to all other drugs. OR, odds ratio; CI, confidence interval; ext, extract; MPFF, micronized purified flavonoid fraction.
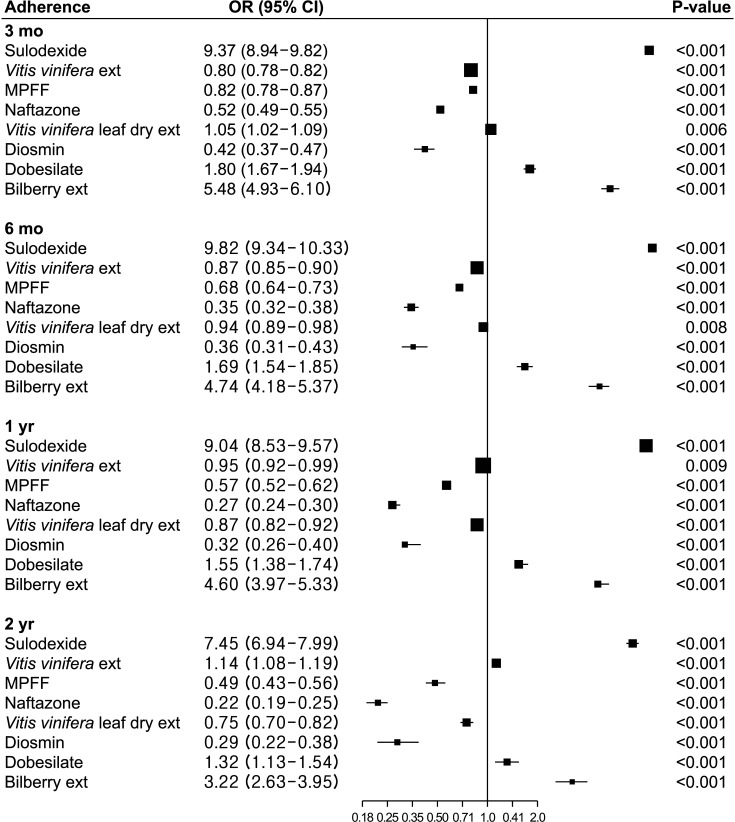
Fig. 3
Heatmap of changed drugs (%) among all started drugs. ext, extract; MPFF, micronized purified flavonoid fraction.
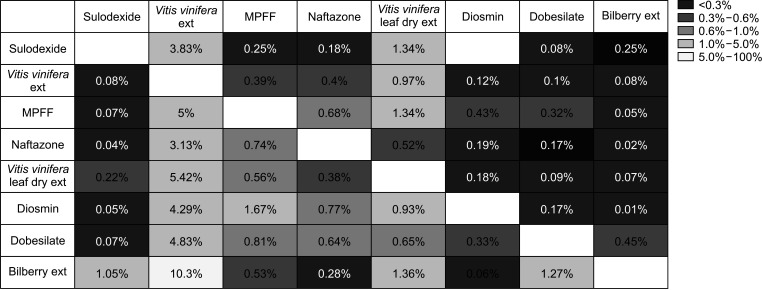
Fig. 4
Adverse events. Each drug with reference to all other drugs. OR, odds ratio; CI, confidence interval; ext, extract; MPFF, micronized purified flavonoid fraction.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download