Abstract
Purpose
Although its efficacy is uncertain, an intraoperative air leak test (ALT) is commonly used to detect mechanical defects following bowel anastomosis. This study aimed to evaluate the efficacy of ALT to detect anastomotic leakage (AL) following rectal excision.
Methods
We reviewed our database for patients with rectal cancers who had undergone curative surgery between January 2012 and January 2018. Patients were grouped according to whether or not an ALT was performed. Propensity score analyses were performed to compare outcomes for groups in a 1:1 case-matched cohort.
Results
In total, 1,191 patients underwent rectal excision; 438 (219 in each group) formed the case-matched cohort for analysis. The protective stoma rate was 16.0% and 14.6% in the ALT and the no-ALT groups, respectively (P = 0.791). In the ALT group, 2 patients (0.9%) showed a positive result and were treated with rectal tube drainage, resulting in no leakage. There was no significant difference in postoperative AL rate between the groups (ALT group: 4.6% vs. no-ALT group: 4.1%, P > 0.999).
Despite the steady improvements in minimally invasive surgery over the past few decades, anastomotic leakage (AL) remains a problem for both patients and physicians [1] as many reports show that AL is associated with poor survival [234]. Surgeons employ various methods to predict and prevent AL following rectal excision. Intraoperative air leak tests (ALTs) are frequently performed to detect mechanical defects in bowel anastomoses. However, the effectiveness of an ALT in the prevention of AL is debatable, as few randomized trials or heterogeneous studies have been performed regarding this subject [56].
ALs have multifactorial causes, including mechanical failure, inadequate blood supply, and infections [6]. Factors that affect bowel anastomotic healing include tissue perfusion, nutritional status, intraperitoneal infections, radiation, blood transfusion, and mechanical bowel preparation [78]. Therefore, there is concern regarding the efficacy of ALTs, which can only identify mechanical defects in anastomoses. Theoretically, an ALT could show false-positive results from applying high-pressure insufflation into the neorectum immediately after an anastomosis [9]. Conversely, low-pressure insufflation could produce false-negative results.
Here, we aimed to evaluate the usefulness of ALT in detecting AL following rectal excision. We hypothesized that performing an ALT would have no significant impact on the prevention of postoperative AL following rectal excision.
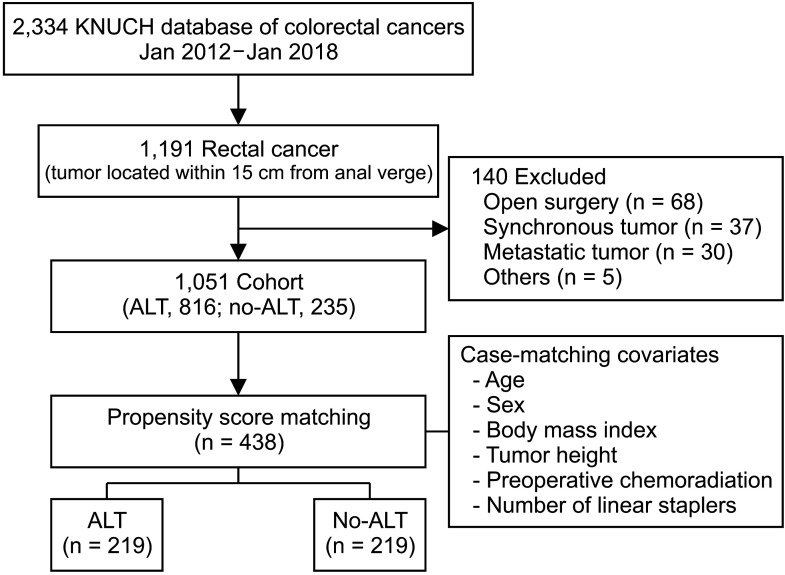
This retrospective study included data from consecutive patients who had undergone surgery for colorectal cancers. We reviewed the database of patients undergoing rectal excision for rectal cancers located within 15 cm of the anal verge (n = 1,191) between January 2012 and January 2018 (Fig. 1). The patients were categorized into 1 of 2 groups based on whether or not an ALT was performed. Patients with a synchronous or metastatic tumor and those who had undergone open surgery were excluded. The entire cohort (n = 1,051) was analyzed (Supplementary Tables 1, 2), and a propensity score analysis was performed; postoperative outcomes were compared for the ALT and no-ALT groups in a 1:1 matched cohort. Covariates for propensity scores included age, sex, body mass index, tumor distance from the anal verge, preoperative chemoradiation status, and the numbers of linear staplers used. Patients with stage T3–4 N0 or node-positive mid- and low-rectal cancers were administered chemoradiotherapy preoperatively. Radiotherapy was administered at a dosage of 50 Gy in 25 fractions for 5 weeks, and the chemotherapy regimens were mainly based on 5-fluorouracil and leucovorin. Surgery on these patients was typically performed 7–9 weeks after their last round of radiotherapy.
This study was performed in line with the principles of the Declaration of Helsinki. All patients provided informed consent, and this study was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital (No. KNUCH 2020–06-034).
All patients underwent mechanical bowel preparation before surgery. They were required to drink 3 L of polyethylene glycol solution after consuming a liquid diet the day before surgery. An enema was performed at 8 pm the day before and at 6 am on the day of surgery. All operations were performed by 4 experienced surgeons. The same principles and steps were applied in both the ALT and no-ALT groups: ligation of the mesenteric vessels close to their origin, mobilization of the sigmoid colon and rectum using sharp dissection with a nerve-sparing technique, and complete splenic flexure takedown for mid- or low-rectal cancers. A rectal division was performed using an endoscopic linear stapler, followed by end-to-end anastomosis using a circular stapler.
In the ALT group, after completing the anastomosis, the pelvis was filled with sterile normal saline solution, and the ALT was performed by inflating air (approximately 50 mL) into the rectum with a syringe. If the ALT was positive, the patient was treated according to the surgeon’s discretion, usually via suture repair, rectal tube drainage, or by creating a diverting stoma. Two surgeons (G.S.C. and. H.J.K.) at our institution usually performed ALTs on their patients after rectal excision. The other 2 (J.S.P. and S.Y.P.) did not do so during the study period. Previously, we reported that male sex, a low anastomosis, preoperative chemoradiation, and multiple firings of linear stapler were risk factors for AL after rectal excision [10]. The 2 surgeons who did not perform ALT instead considered the creation of a diverting stoma for high-risk patients with 2 or more risk factors. In the no-ALT group, the surgeon mainly employed a digital rectal examination to identify the anastomosis site. In particular, an intraoperative colonoscopy was performed to evaluate anastomosis in patients with anal bleeding after stapled anastomosis. The assistant surgeon, who had been trained in colonoscopy for several years, stood between the legs of the patient. The laparoscopic and colonoscopic monitors were placed side-by-side on the patient’s left side (Supplementary Fig. 1), enabling the surgeon to inspect both monitors simultaneously. The pelvic cavity was filled with sterile normal saline solution and the bowel was clamped gently about 15 cm above the anastomosis using a surgical grasper. A flexible colonoscope was gently inserted through the anus. The anastomosis line was evaluated for defects, bleeding, and ischemic change. Air insufflation with low pressure within 2 seconds was performed at the anastomosis level (Supplementary Video 1).
AL was defined as a defect in the intestinal wall at the anastomosis site along with the manifestation of clinical symptoms such as fever or abdominal pain, pelvic abscess, peritonitis, and drainage of pus from the pelvic drain. A major leakage was defined as when the patient exhibited peritonitis and sepsis requiring emergency reoperation. AL was usually confirmed by physical examination, abdominal CT, or during reoperation. Minor leakages were treated without reoperation. Postoperative complications were classified according to the Clavien-Dindo scheme [11]. Stoma-free survival time was calculated from the initial surgery or closure of an ileostomy to the time of stoma creation for any reason, or to the last outpatient visit.
Continuous variables were first evaluated for normality using the Shapiro-Wilk test. Both groups were compared using a 2-sample Student t-test or Kruskal-Wallis rank-sum test; categorical variables were assessed using a chi-squared test or Fisher exact test. Propensity score matching was applied to minimize selection bias and reduce differences between the 2 groups. A multivariate logistic regression analysis was performed to obtain propensity scores. Covariates for the propensity scores included age, sex, body mass index, tumor distance from the anal verge, preoperative chemoradiation status, and the number of linear staplers used. These covariates are well-known risk factors for postoperative AL following rectal excision. Multivariable analysis was conducted using logistic regression to identify independent risk factors for AL. Variables with a P-value of <0.2 in the univariate analysis and the incidence of ALT were selected for the multivariable analysis. In addition, well-known risk factors were selected for the multivariable analysis [10]. The Kaplan-Meier method was used to compare stoma-free survival among the patients. All analyses were conducted using the R Project for Statistical Computing, ver. 3.9.1 (R Foundation for Statistical Computing; https://www.r-project.org), and the P-value of <0.05 was considered statistically significant.
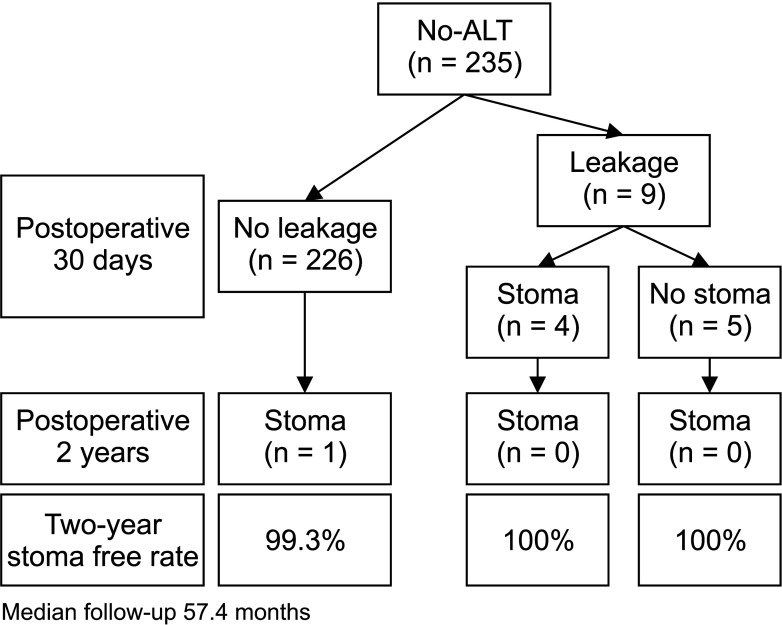
The ALT and no-ALT groups comprised 816 and 235 patients, respectively (Supplementary Table 1). Of the 816 patients with ALTs, 0.9% showed positive results. AL rates were similar in both groups (ALT vs. no-ALT, 3.1% vs. 3.8%). The mean time taken to diagnose the AL after surgery was 4.2 days (range, 1–16 days). In the no-ALT group, the 2-year stoma-free rates during the median follow-up period of 57.4 months were 99.3% and 100% in patients without and with AL, respectively (Fig. 2). One patient had a permanent stoma, not because of an anastomotic problem with the anastomosis but because of a functional problem.
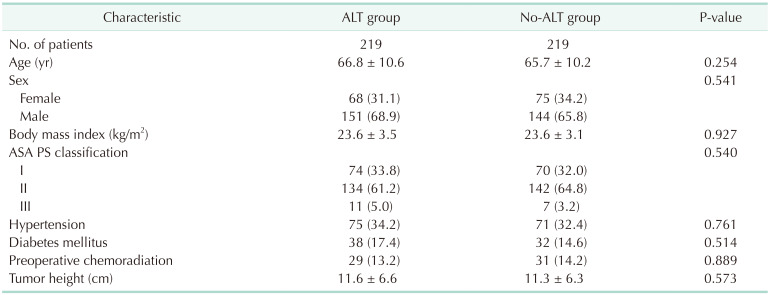
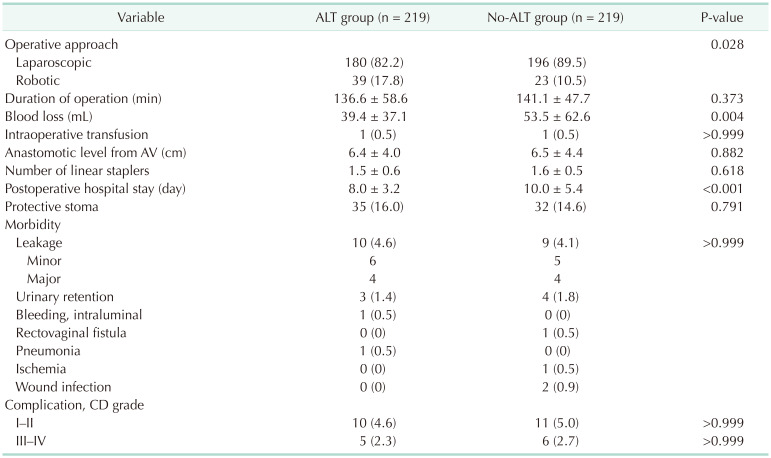
After propensity score matching, 219 patients were allocated to each group; there were no considerable differences in any of the covariates in the matched cohort (Table 1). Operative and postoperative details of the 2 groups are shown in Table 2. The no-ALT group was associated with a higher degree of blood loss than the ALT group (53.5 mL vs. 39.4 mL, P = 0.004). The anastomotic level from the anal verge was similar in both groups. The no-ALT group showed a significantly longer postoperative hospital stay than the ALT group (10.0 days vs. 8.0 days, P < 0.001).
The postoperative AL rate was 4.6% and 4.1% in the ALT and no-ALT groups, respectively (P > 0.999). There were no significant differences in the incidence of other morbidities between the groups. The rates of major complications (Clavien-Dindo complication grade of >III) were similar in both the ALT (2.3%) and no-ALT groups (2.7%) (P > 0.999).
In the ALT group, 2 patients (0.9%) showed positive ALT results and were treated using rectal tube drainage. These patients did not require reoperation during the 30-day postoperative period. In the ALT group, 10 patients had AL; a diverting stoma was created in 4 of them. The remaining 6 were treated using rectal tube drainage without creating a stoma. In the no-ALT group, 9 patients had AL, and a diverting stoma was created in 4 of them; the other 5 were treated using rectal tube drainage without creating a stoma.
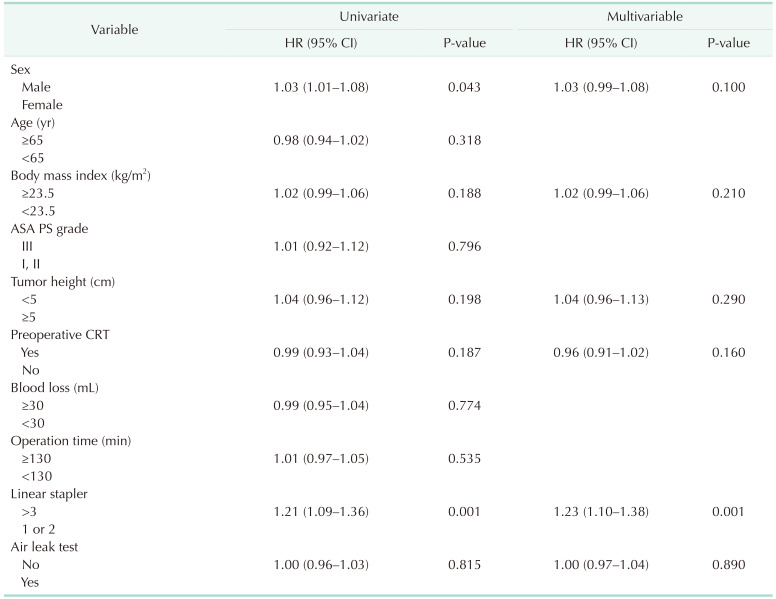
Univariate analyses were performed for potentially associated variables to identify the risk factors for AL (Table 3). Male sex and multiple firings (>3) of the linear stapler were significantly associated with AL. In multivariable analyses, multiple firings (>3) of the linear stapler were an independent risk factor for AL. Among patients who underwent ALT in the entire cohort, 24 with a negative ALT eventually developed a leak (false-negative rate of 96.0%). The ALT was positive in 0.9% of the patients.
This study aimed to examine the usefulness of ALTs in detecting AL following rectal excision. The results showed that the AL rate was not significantly different between patients who underwent ALT and those who did not. Furthermore, the ALT positive rate was 0.9%, and we observed a false-negative rate of 96.0%. These results indicated that ALT was not enough to detect AL and reduce the rate of AL following rectal excision.
Performing an ALT is a popular technique for identifying anastomotic line defects during surgery. Although many surgeons expect that a negative ALT result indicates zero or lower risk of postoperative leaks, it gives a false sense of the possibility of an AL. In this study, it is of concern that 96.0% of those diagnosed with AL in the ALT group (24 of 25 patients) had initially shown negative results with the test. There are some potential reasons why the ALTs failed to reduce the incidence of AL following rectal excision. The main reason could be the lack of association between ALTs and AL, as most of the patients with an AL presented a few days or weeks after the surgery. In this study, the mean number of days taken to detect a leak postsurgery was 4.2 days (range, 1–16 days). Daams et al. [12] reported a mean of 8 postoperative days for colorectal AL to become clinically apparent. Considering the physiology of wound healing, Munireddy et al. [7] reported that the anastomosis was at risk of leakage or dehiscence in the first 3–10 postoperative days because of collagen lysis caused by collagenase activity. This explains the high false-negative rate of intraoperative ALT.
The second reason could be that an intraoperative ALT is only used to detect anastomotic line defects that could result from a rare technical error or stapler misfiring. This eventuality is very rare in high-volume centers with operations performed by well-experienced surgeons; however, the rate of stapler misfiring is unclear in the literature. Nonetheless, Trencheva et al. [13] reported that the rate of intraoperative surgical complications was 1.95%, including injury to other organs, bleeding, and stapling malfunction. Even in the case of bariatric surgery, an intraoperative ALT could not detect mechanical insufficiency, even when the surgeon was aware of a stapler misfire and an AL developing subsequently [14]. Mizrahi et al. [15] reported that an intraoperative leakage was evident in only 1 of 4 patients with the defect found later by CT. It was speculated that symptomatic micro leaks or tissue edema at the leak site could be responsible for the negative results obtained in intraoperative ALTs.
There are some concerns regarding intraoperative ALTs, as there might be an immediate reduction in anastomotic strength after anastomosis. Although there are no published descriptions of the incidence of any adverse event following an ALT, there are some theoretical concerns involving factors such as the physiology of anastomotic healing described by Thompson et al. [8]. Anastomotic strength is low during the first few postoperative days. Therefore, there is a risk of barotrauma immediately following the anastomosis caused by excess pressure of the injected air. However, it is difficult to define the optimal volume and pressure of injected air in an ALT. In an in vitro study, newly constructed colorectal anastomoses were observed to burst at pressures of 70–184 mmHg [16]. Due to variations in the anatomy and physiology of patients, it is difficult to determine the optimal volume or pressure of the injected air. Excessive pressure can cause false-positive results, whereas lower pressure can produce false-negative results.
In the no-ALT group, the surgeon assessed the anastomosis via digital rectal examination. Creating a diverting stoma was considered based on a combination of the risk factors described in a previous study [10], and rectal tube drainage was employed in patients with intermediate risk [17]. Therefore, the postoperative hospital stay of this group was longer than in the ALT group. Further research is required to investigate the effect of rectal tube drainage compared with the creation of a stoma.
This is not the first study evaluating the efficacy of ALT following colorectal anastomosis. Schmidt et al. [18] reported that intraoperative anastomotic testing failed to improve the rate of AL. Wheeler and Gilbert [19] proposed that an intact, leakage-tested anastomosis did not guarantee that the anastomosis would remain intact postoperatively. Some authors have expressed their concerns about the false-negative results of ALTs. Pritchard et al. [20] argued that intraoperative testing could be misleading because AL was not predicted in 18% of patients based on intraoperative ALTs. Ricciardi et al. [21] reported that an airtight anastomosis did not guarantee postoperative anastomotic integrity. A recent meta-analysis showed that ALT did not significantly reduce the clinical AL rate [6]. Some studies have reported the absence of any correlation between the incidence of intraoperative ALTs and AL after bariatric surgery [142223]. However, other studies have shown that an ALT helps prevent AL. Following those reports, Davies et al. [24] proposed that the ALT was simple to perform and probably helped reduce postoperative AL. A randomized controlled trial demonstrated that an ALT reduced the risk of AL [25]. Allaix et al. [26] reported that intraoperative ALTs enable the detection of AL defects after laparoscopic left-sided colon resection, which could be effectively managed intraoperatively, leading to a significantly lower risk of postoperative AL. Previous studies have shown higher preoperative chemoradiotherapy rates (52.3%–67.4%) than those reported in our study [2132627]. The main reason was that we omitted preoperative chemoradiotherapy in higher rectal cancer [28]. Moreover, previous studies conducted in Asian countries have shown relatively lower preoperative chemoradiotherapy rates (12.8%–28.6%) [102930]. Therefore, our results should be cautiously interpreted.
Intraoperative endoscopy is an option for assessing an anastomosis using direct vision [30]. In a systematic review, the positive results of colonoscopy were related to a high risk of AL [5]. Surgeons were able to evaluate stapling failure and bleeding and identify mucosal ischemia via direct visualization [30]. However, there are still concerns that high air pressure or abnormal scope of movement might harm the anastomosis. Therefore, intraoperative endoscopy should be only performed by experienced surgeons.
This study had several limitations. First, there might have been a bias in patient and treatment selection because this study was retrospective in design. Although an attempt was made to create 2 validly comparable groups by propensity score matching for well-known AL risk factors, concerns about hidden confounding factors remain. Second, individual factors among the surgeons might also have influenced the results. This is because ALTs were only performed by 2 of the 4 surgeons in our institution, although both were experienced and had performed more than 200 minimally invasive colorectal cancer operations per year. In addition, case matching was performed to reduce bias. To our knowledge, this is the first propensity score-matched analysis evaluating the efficacy of ALT following rectal excision.
In conclusion, the current ALT method might not be useful for detecting AL following rectal excision because the positive rate of ALT was low, and the false-negative rate of ALT was high. Further studies are warranted to clarify whether AL can be prevented by ALT or alternative methods.
Notes
Fund/Grant Support: This work was supported by a Biomedical Research Institute grant, Kyungpook National University Hospital (2018) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
References
1. Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. 2014; 18:1176–1185. PMID: 24671472.
2. Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol. 2015; 22:2059–2067. PMID: 25348782.
3. McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005; 92:1150–1154. PMID: 16035134.
4. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011; 253:890–899. PMID: 21394013.
5. Nachiappan S, Askari A, Currie A, Kennedy RH, Faiz O. Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc. 2014; 28:2513–2530. PMID: 24718665.
6. Wu Z, van de Haar RC, Sparreboom CL, Boersema GS, Li Z, Ji J, et al. Is the intraoperative air leak test effective in the prevention of colorectal anastomotic leakage? A systematic review and meta-analysis. Int J Colorectal Dis. 2016; 31:1409–1417. PMID: 27294661.
7. Munireddy S, Kavalukas SL, Barbul A. Intra-abdominal healing: gastrointestinal tract and adhesions. Surg Clin North Am. 2010; 90:1227–1236. PMID: 21074038.
8. Thompson SK, Chang EY, Jobe BA. Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery. 2006; 26:131–136. PMID: 16518804.
9. Kim JH, Kim HY, Lee IK, Oh ST, Kim JG, Lee YS. Intra-operative double-stapled colorectal or coloanal anastomotic complications of laparoscopic low anterior resection for rectal cancer: double-stapled anastomotic complication could result in persistent anastomotic leakage. Surg Endosc. 2015; 29:3117–3124. PMID: 25519426.
10. Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013; 257:665–671. PMID: 23333881.
11. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID: 19638912.
12. Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: a systematic review of literature. World J Gastrointest Surg. 2014; 6:14–26. PMID: 24600507.
13. Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T, et al. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg. 2013; 257:108–113. PMID: 22968068.
14. Bingham J, Lallemand M, Barron M, Kuckelman J, Carter P, Blair K, et al. Routine intraoperative leak testing for sleeve gastrectomy: is the leak test full of hot air? Am J Surg. 2016; 211:943–947. PMID: 27020902.
15. Mizrahi I, Tabak A, Grinbaum R, Beglaibter N, Eid A, Simanovsky N, et al. The utility of routine postoperative upper gastrointestinal swallow studies following laparoscopic sleeve gastrectomy. Obes Surg. 2014; 24:1415–1419. PMID: 24737310.
16. Schwab R, Wessendorf S, Gutcke A, Becker P. Early bursting strength of human colon anastomoses: an in vitro study comparing current anastomotic techniques. Langenbecks Arch Surg. 2002; 386:507–511. PMID: 11819108.
17. Yang CS, Choi GS, Park JS, Park SY, Kim HJ, Choi JI, et al. Rectal tube drainage reduces major anastomotic leakage after minimally invasive rectal cancer surgery. Colorectal Dis. 2016; 18:O445–O452. PMID: 27611180.
18. Schmidt O, Merkel S, Hohenberger W. Anastomotic leakage after low rectal stapler anastomosis: significance of intraoperative anastomotic testing. Eur J Surg Oncol. 2003; 29:239–243. PMID: 12657233.
19. Wheeler JM, Gilbert JM. Controlled intraoperative water testing of left-sided colorectal anastomoses: are ileostomies avoidable? Ann R Coll Surg Engl. 1999; 81:105–108. PMID: 10364966.
20. Pritchard GA, Krouma FF, Stamatakis JD. Intraoperative testing of colorectal anastomosis can be misleading. Br J Surg. 1990; 77:1105. PMID: 2224456.
21. Ricciardi R, Roberts PL, Marcello PW, Hall JF, Read TE, Schoetz DJ. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg. 2009; 144:407–412. PMID: 19451481.
22. Sethi M, Zagzag J, Patel K, Magrath M, Somoza E, Parikh MS, et al. Intraoperative leak testing has no correlation with leak after laparoscopic sleeve gastrectomy. Surg Endosc. 2016; 30:883–891. PMID: 26092015.
23. Bingham J, Kaufman J, Hata K, Dickerson J, Beekley A, Wisbach G, et al. A multicenter study of routine versus selective intraoperative leak testing for sleeve gastrectomy. Surg Obes Relat Dis. 2017; 13:1469–1475. PMID: 28629729.
24. Davies AH, Bartolo DC, Richards AE, Johnson CD, McC Mortensen NJ. Intra-operative air testing: an audit on rectal anastomosis. Ann R Coll Surg Engl. 1988; 70:345–347. PMID: 3207322.
25. Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg. 1990; 77:1095–1097. PMID: 2136198.
26. Allaix ME, Lena A, Degiuli M, Arezzo A, Passera R, Mistrangelo M, et al. Intraoperative air leak test reduces the rate of postoperative anastomotic leak: analysis of 777 laparoscopic left-sided colon resections. Surg Endosc. 2019; 33:1592–1599. PMID: 30203203.
27. Brisinda G, Chiarello MM, Pepe G, Cariati M, Fico V, Mirco P, et al. Anastomotic leakage in rectal cancer surgery: retrospective analysis of risk factors. World J Clin Cases. 2022; 10:13321–13336. PMID: 36683625.
28. Park JS, Sakai Y, Simon NS, Law WL, Kim HR, Oh JH, et al. Long-term survival and local relapse following surgery without radiotherapy for locally advanced upper rectal cancer: an international multi-institutional study. Medicine (Baltimore). 2016; 95:e2990. PMID: 27258487.
29. Wu Y, Zheng H, Guo T, Keranmu A, Liu F, Xu Y. Temporary diverting stoma improves recovery of anastomotic leakage after anterior resection for rectal cancer. Sci Rep. 2017; 7:15930. PMID: 29162894.
30. Yang SY, Han J, Han YD, Cho MS, Hur H, Lee KY, et al. Intraoperative colonoscopy for the assessment and prevention of anastomotic leakage in low anterior resection for rectal cancer. Int J Colorectal Dis. 2017; 32:709–714. PMID: 28144745.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1, 2, Supplementary Fig. 1, and Supplementary Video 1 can be found via https://doi.org/10.4174/astr.2023.104.4.214.
Supplementary Fig 1
Position of the surgeon, assistant endoscopic surgeon, and monitors during intraoperative colonoscopy.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download