Abstract
Purpose
The prognostic significance and treatment of lateral pelvic lymph node metastasis (mLPLN) in rectal cancer patients receiving neoadjuvant chemoradiotherapy (nCRT) are not well understood. In this study, we evaluated the impact of mLPLN identified in imaging modality on outcomes.
Methods
Between January 2008 and December 2016, 1,535 patients who underwent radical resection following nCRT were identified. The association between mLPLN and disease-free survival (DFS), overall survival (OS), local recurrence-free survival (LRFS), and pelvic recurrence-free survival (PRFS) was analyzed, along with risk factors associated with OS and DFS.
Results
Overall, 329 (21.4%) of the 1,535 patients experienced disease recurrence; 71 (4.6%) had local recurrence, 25 (1.6%) had pelvic recurrence, and 312 (20.3%) had distant recurrence. The pre- and post-nCRT mLPLN (–) groups had better DFS, LRFS, PRFS, and OS than the (+) groups. LPLN sampling (LPLNs) was implemented in 24.0% of the pre-nCRT mLPLN (+) group and in 28.8% of the post-nCRT mLPLN (+) group. There was no significant difference in OS and LRFS between LPLNs group and no LPLNs group in pre- and post-nCRT mLPLN (+) groups. Pre-nCRT mLPLN was associated with poor OS (hazard ratio [HR], 1.43; P = 0.009) and post-nCRT mLPLN was associated with poor DFS (HR, 1.49; P = 0.002).
Although the total mesorectal excision (TME) and neoadjuvant chemoradiotherapy (nCRT) have significantly improved local control of rectal cancer [123456], local recurrence (LR) is still a bothersome issue for patients with rectal cancer. Previous studies identified several risk factors for LR such as female sex, clinical T stage, and pathologic T and N stage, and lymph node metastasis is a well-known significant risk factor for both LR and distant metastasis [7]. The lateral pelvic lymph node (LPLN) is one of the main LR sites. The LPLN metastasis (mLPLN) occurred in 10%–25% of low rectal cancer patients [8], and 7% of such cases involve hidden micrometastases in lymph nodes that are undetectable by conventional histopathology [9]. Furthermore, up to 15% of patients metastases in the LPLN despite the absence of positive nodes along the inferior mesenteric artery [10]; these metastases are associated with tumor location and stage [11121314].
Therefore, we expected that removing suspicious metastatic LPLN can prevent LR. According to Japanese studies, LPLN dissection (LPLND) has a stronger therapeutic value than traditional lymphadenectomy [10]. However, Western countries (and South Korea) considered mLPLN except for internal iliac artery lymph node as distant metastases [15], and nCRT and TME is the standard treatment for locally advanced rectal cancers.
As a result, the necessity for the LPLND has not received much attention since the introduction of nCRT except in some research groups in surgical society other than Japanese group. However, in recent years, studies reported that the lateral pelvic wall is the primary site of LR even following nCRT, and Western countries have shown more interest [1617181920]. Indeed, recent studies show that nCRT and TME are insufficient and that LPLND may be beneficial in subgroup of patients. However, the oncologic significance of mLPLN is still unclear.
We believe that the primary reason for the debate surrounding treatment indications is the unclear oncologic implications of mLPLN in pre- and post-nCRT status. Therefore, we aimed to identify independent risk factors for recurrence or survival, as well as the oncologic outcomes of rectal cancer patients treated with nCRT according to mLPLN diagnosed at pre- and post-nCRT status.
The study protocol of this study was approved by the Institutional Review Board of Asan Medical Center (No. 2020-0346). The study was performed in accordance with the Declaration of Helsinki and the requirement for informed patient consent was waived due to the retrospective design of the study.
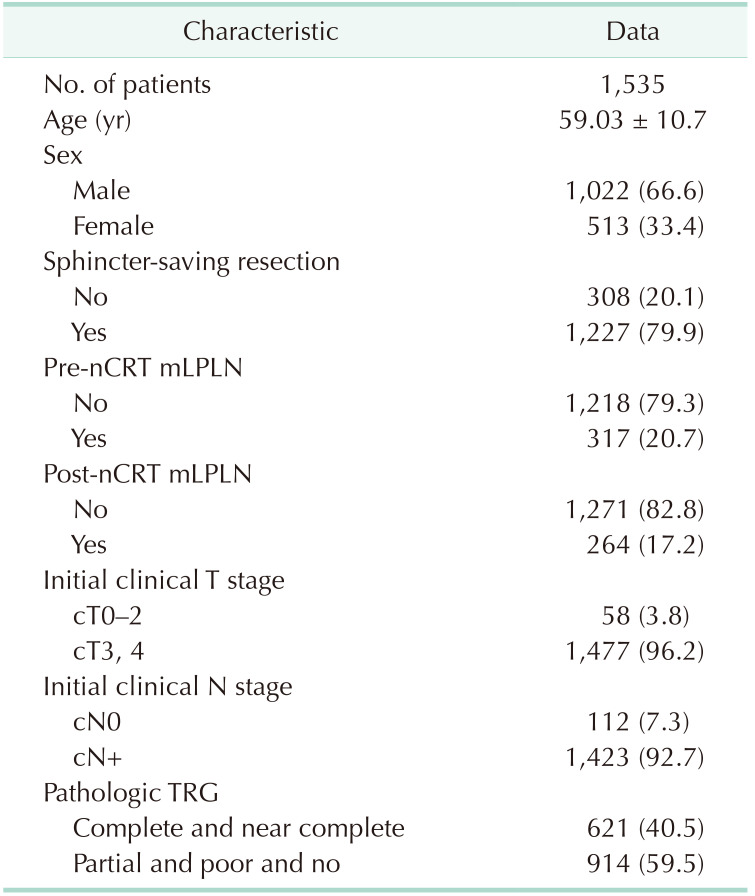
The study included 1,535 patients who received nCRT and TME between January 2008 and December 2016 at Asan Medical Center in Seoul, Korea. Patient records were reviewed retrospectively to identify clinicopathologic features, LPLN status prior to and following nCRT, disease recurrence, and survival status.
Eligibility criteria included rectal cancer within 15 cm of the anal verge treated with nCRT, and patients with pelvic MRI data regarding pre- and postoperative LPLN status. Patients with concurrent distant metastasis at diagnosis, those with concurrent or prior malignancies within 5 years of rectal cancer diagnosis, or who had received prior immunotherapy or radiotherapy to the pelvis, were excluded. In addition, patients were excluded if they did not receive surgical treatment, if there was no available pre- and posttreatment MRI, or if the pathologic stage following treatment could not be determined. Patients with hereditary nonpolyposis colorectal cancer or familial adenomatous polyposis were also excluded (Fig. 1).
Prior to nCRT, all patients were evaluated by digital rectal examination, blood test (including CEA levels), colonoscopy, and abdominopelvic CT with MRI. mLPLN was defined as an enlarged lymph node with a short axis (SA) of >5 mm on pre- or post-nCRT MRI, or as radiologic malignant characteristics such as a round, spiculated, ill-defined margin or a heterogeneous signal (Fig. 2).
Patients with rectal cancer diagnosed as clinically T3 or clinically node-positive without distant metastasis with a threatened circumferential resection margin of <1 mm on MRI, were recommended for nCRT. Some patients with low rectal cancer with ≤cT2 disease received nCRT to shrink the tumor field to save the anal sphincter. About 4–6 weeks after completing nCRT, all patients underwent colonoscopy or sigmoidoscopy, pelvic MRI, and/or transrectal ultrasonography to evaluate treatment response.
During the preoperative chemoradiotherapy treatment period, a dose of 45–50.4 Gy of radiation was administered in 25–28 fractions to a target volume comprising the main tumor, the perirectal adipose tissue, the lateral pelvis, and the presacral lymph nodes. Patients received concurrent capecitabine, 5-fluorouracil (5-FU)/leucovorin (LV), or 5-FU/LV/oxaliplatin (FOLFOX) with radiotherapy. Beginning on the 1st day of irradiation, patients received twice-daily oral capecitabine (825 mg/m2/day), which was continued throughout the entire radiation period. Intravenous 5-FU (375 mg/m2/day) and LV (20 mg/m2/day) was administered during the 1st and 5th weeks of nCRT.
At 6–10 weeks following completion of nCRT, all patients underwent curative resection based on the principle of tumor-specific mesorectal excision. LPLN sampling (LPLNs) of lymph nodes suspected of harboring metastases on pre- or post-MRI was recommended. Whether to perform LPLNs was usually decided based on post-nCRT MRI findings. The decision to perform LPLNs was made after discussion between the surgeon and the radiologist considering primary tumor status, treatment response, and radiologic features. For some patients, however, it was decided to perform LPLNs based on pre-nCRT MRI images according to surgeons’ preference.
Pathologists specializing in colorectal malignancy examined postsurgical specimens to evaluate the tumor response. Pathologic complete/near complete regression was deemed to be good response, while partial/poor or no regression was regarded as a poor response.
All medically fit patients who underwent nCRT were recommended to receive adjuvant chemotherapy. The recommended adjuvant regimen comprised 4 cycles of 5-FU and LV monthly or 6 cycles of capecitabine for patients with ypT0–2/N– disease, and 8 cycles of FOLFOX for those with ypT3/4 or N+ disease.
Following surgery, patients were followed up every 3–12 months for up to 5 years. Physical examination, blood tests including CEA levels, and abdominopelvic CT and/or chest CT were all part of the follow-up evaluation. Patients underwent colonoscopy 1 year after surgical resection, and then every 2–3 years thereafter. Patients with preoperative obstructive lesions underwent colonoscopy within 6 months after surgical resection. Additional imaging procedures such as PET-CT or liver MRI were carried out when worrisome lesions were noted during regular surveillance imaging (i.e., abdominopelvic CT or chest CT).
LR was defined as recurrence with clinical, radiologic, or endoscopic evidence of an intraluminal tumor in the region immediately surrounding the primary resection site, or as a tumor within the mesorectum or rectal wall following the initial operation. Recurrence in organs such as the liver, lung, peritoneum, and bone was referred to as distant recurrence (DR). Recurrence in the pelvic cavity alone, comprising the common iliac, external iliac, internal iliac, and obturator lymph nodes, was defined as pelvic recurrence (PR). Neither LR nor DR includes PR. Distant lymph nodes not categorized as PR were categorized as DR.
Imaging results were used to determine recurrence; patients with ambiguous imaging results underwent ongoing serial change monitoring. To diagnose recurrence, a tissue biopsy was obtained if practical.
The primary endpoints for this study were disease-free survival (DFS), defined as the interval from surgery to tumor recurrence, and overall survival (OS), defined as the interval from surgery to death from any cause (not just cancer-related death) or the last date of data assessment. Local recurrence-free survival (LRFS), which is the interval between the operation and an LR, and pelvic recurrence-free survival (PRFS), which is the interval between the operation and a PR, were secondary endpoints. The associations between pre- and post-nCRT and these endpoints were assessed. Categorical and continuous variables were assessed using the chi-square test and t-test, respectively. DFS, LRFS, PRFS, and OS, were assessed using the Kaplan-Meier method and the log-rank test. Age, sphincter-saving resection, initial clinical T stage, lymphovascular invasion (LVi), perineural invasion (PNi), and pre- and post-mLPLN were compared as risk factors for OS and DFS using a multivariable analysis with Cox proportional hazards model. The P-values of <0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics ver. 21.0. (IBM Corp.).
Pre-nCRT mLPLN was found in 317 (20.7%) of the 1,545 patients, while post-nCRT mLPLN was found in 264 (17.2%) of all patients (Table 1).
The pre-nCRT mLPLN (+) group had higher rates of LVi (15.1% vs. 10.2%, P = 0.022), PNi (20.2% vs. 13.4%, P = 0.006), and lower rates of sphincter preservation (70.3% vs. 82.4%, P < 0.001) than pre-nCRT mLPLN (–) group. Patients with post-nCRT mLPLN (+) also had higher LVi and PNi. Sphincter preservation was less common in those with post-nCRT mLPLN (+). A good response to nCRT was less common in post-nCRT mLPLN (+) group than post-nCRT mLPLN (–) group (36.7% vs. 41.2%) (Supplementary Table 1), but the difference was not significant.
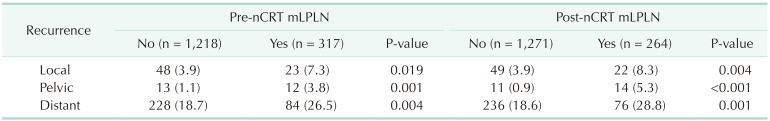
Of the 1,535 patients, 329 (21.4%) experienced disease recurrence; 71 (4.6%) had LR, 25 (1.6%) had PR, and 312 (20.3%) had DR. The pre-nCRT mLPLN (+) group had a considerably higher LR rate than the (–) group (7.3% vs. 3.9%, P = 0.019). Similarly, the pre-nCRT mLPLN (+) group had a higher incidence of DR than the (–) group (26.5% vs. 18.7%, P = 0.004). Recurrence rates also differed according to post-nCRT mLPLN status. The post-nCRT mLPLN (+) group showed higher rates of LR (8.3% vs. 3.9%, P = 0.004) and DR (28.8% vs. 18.6%, P = 0.001) than the (–) group (Table 2).
PR occurred in 13 (1.1%) patients in the pre-nCRT mLPLN (–) group and in 12 (3.8%) patients in the pre-nCRT mLPLN (+) group (P = 0.001). The percentage of patients with PR in the post-nCRT mLPLN (+) group was higher than that in the post-nCRT mLPLN (–) group (5.3% vs. 0.9%, P < 0.001).
DFS, OS, LRFS, and PRFS for the overall cohort were 75.7%, 67.1%, 92.9%, and 97.9%, respectively. Patients in the pre-nCRT mLPLN (+) group had lower 5-year DFS (69.9% vs. 79.7%), OS (77.1% vs. 86.1%), LRFS (92.2% vs. 96.3%), and PRFS (96.5% vs. 98.9%) than those in the pre-nCRT mLPLN (–) group (Fig. 3). The 5-year DFS, OS, LRFS, and PRFS were also poorer for patients in the post-nCRT mLPLN (+) group than those in the post-nCRT mLPLN (–) group (DFS, 67.6% vs. 79.8%; OS, 78.5 vs. 85.0%; LRFS, 91.6% vs. 96.4%; and PRFS, 81.4% vs. 94.6%) (Fig. 4).
In univariable analysis, the initial clinical N stage was not significantly related to DFS or OS (Supplementary Table 2). Pathologic stage is a significant factor for both DFS and OS, but it showed a significant correlation with pathologic tumor regression grade (TRG; r = 0.462, P < 0.001); therefore, only pathologic TRG was included in the multivariable analysis.
Multivariable analysis identified sphincter-saving resection, initial clinical T stage, PNi, and pathologic TRG being associated with OS and DFS. Pre-nCRT mLPLN was associated with OS, but post-nCRT mLPLN was associated with DFS (Table 3).
We evaluated risk factors associated with distant metastasis, LR, and PR (Supplementary Tables 3, 4, 5). Patholgoic stage and TRG were significantly correlated and we included TRG in multivariable analysis when both of them showed significance in univariable analysis. In the multivariable analysis, pre-nCRT mLPLN was not related to local, pelvic, or distant metastasis. However, post-nCRT mLPLN was associated with PR but not with local or distant metastasis.
Of the 1,535 patients, 97 (6.3%) underwent LPLNs. In total, 412 lymph nodes were harvested and more than 60% of patients who underwent LPLNs yielded fewer than 5. In 11 patients (11.6%) who underwent LPLNs, there was no LPLN in pathologic examination.
Among the 97 patients who underwent LPLNs, pathologic examination confirmed mLPLN in 28 (28.9%). Two patients who were classified as mLPLN (–) received LPLNs due to an equivocal finding of mLPLN on pre- and post-nCRT MRI; a final pathologic examination showed that they had mLPLN.
Overall, 76 (24.0%) of the 317 patients in the pre-nCRT mLPLN (+) group underwent LPLNs, of whom 24 (31.6%) had pathologically proven mLPLN. In the post-nCRT mLPLN (+) group, 76 (28.8%) of the 264 patients underwent LPLNs, of whom 24 (31.6%) had pathologically proven mLPLN.
Among patients in the pre-nCRT mLPLN (+) group, the 5-year DFS rate was higher in those who did not undergo LPLNs than in those who did (72.8% vs. 60.7%, P = 0.035). For patients in the post-nCRT mLPLN (+) group, the 5-year DFS rate in the no LPLNs group was also higher (70.3%) than that in the LPLNs group (60.7%), although this difference was not statistically significant (P = 0.129). In both the pre-nCRT mLPLN (+) and post-nCRT mLPLN (+) groups, OS and LRFS were not different according to LPLNs. PRFS was higher in the no LPLNs group in the pre-nCRT and post-nCRT mLPLN (+) groups (Supplementary Fig. 1).
Recent studies showed that, despite nCRT with TME improving oncologic outcomes, patients with rectal cancer who have mLPLN have poor outcomes [2122], and patients with locally advanced rectal cancer have a 10%–25% chance of developing mLPLN [2324].
We found that patients in the pre- or post-nCRT mLPLN (+) groups had greater rates of LR, PR, and DR, and lower rates of DFS, OS, LRFS, and PRFS, respectively. Furthermore, pre-nCRT mLPLN (+) was a risk factor for OS, whereas post-nCRT mLPLN (+) was a risk factor for DFS. This suggests that pre-and post-nCRT mLPLN (+) are important predictors of oncologic outcome, but their effects are different. Here, we focused on the variations in the effects of mLPLN (+) before and after nCRT.
Numerous studies have examined the oncologic effects of mLPLN. Most demonstrate that mLPLN is associated with LR [11121314]; however, other studies demonstrated that mLPLN is also associated with DR. Kim et al. [25] showed that patients with pre-nCRT mLPLN (+) with SA of ≥7 mm had poorer LRFS, PRFS, and DR-free survival than those with pre-nCRT mLPLN (–). In addition, Zhou et al. [26] investigated 141 rectal cancer patients with clinical evidence of mLPLN who underwent TME + LPLND and divided them into an mLPLN group and a non-mLPLN group based on pathologic reports. They found that mLPLN was an independent risk factor for both OS and DFS in patients with mLPLN following TME + LPLND.
The results of this study suggest that depending on the situation, we should determine the importance of mLPLN. It seems appropriate to base a choice on the results of post-nCRT for disease control. Additionally, the treatment should be intensified once pre-nCRT mLPLN is identified even when the post-nCRT mLPLN shrinks; this is because pre-nCRT mLPLN has a detrimental effect on OS and it should be considered as risk factor for a poor prognosis.
The imaging modalities and size criteria used to diagnose LPLN vary from study to study. In addition, it is not clear whether LPLN size pre- or post-nCRT is a better criterion. Each center has a different size standard. Recent findings from a large Western study conducted by the MD Anderson Cancer Center suggested that patients with rectal cancer and a post-nCRT LPLN SA of ≥5 mm ought to be considered for mLPLN [19]. The Lateral Node Study Consortium revealed that the LPLND significantly reduced 5-year lateral PR and DR rates in patients with an LPLN SA of >7 mm on pre-nCRT MRI [11]. Kim et al. [25] found that patients in whom the LPLN SA diameter decreased from ≥7 mm on pre-nCRT MRI to <4 mm after nCRT had a lower incidence of LR; however, the degree of DR risk in 798 rectal cancer patients treated with nCRT remained the same. In the Netherlands, radiologists proved that in addition to size, other parameters, such as speculated border and heterogeneous signal, may be effective in predicting regional lymph node involvement in patients with rectal cancer [27]. In the present study, we examined both tumor size and features and characterized LPLN as an enlarged lymph node with a SA of >5 mm on MRI, or with malignant radiologic features.
Historically, LPLND was recommended for the treatment of mLPLN occurring after nCRT; however, LPLND requires a sensitive and complex technique and can result in several morbidities, including substantial blood loss, urinary retention, and sexual dysfunction. Lee at el. [28] evaluated patients who underwent TME + LPLND versus TME alone after nCRT. The LPLND group had significantly longer operating time (562 minutes vs. 436 minutes, P = 0.015), and more blood loss (560 mL vs. 135 mL, P = 0.05) than the TME alone group. JCOG0212 (Japan Clinical Oncology Group Study 0212) showed similar results [29]. In this study, patients who underwent LPLND had significantly longer operating time (360 minutes vs. 254 minutes, P < 0.001) and greater blood loss (576 mL vs. 337 mL, P < 0.001). In addition, while identifying patients who are most suitable for LPLND is essential for achieving oncologic benefits, screening such patients is challenging. The size of LPLN before and after nCRT is one factor used to determine LPLND, but (as mentioned above) the size requirement differs according to the institution.
Our center does not perform LPLND routinely for low rectal cancer patients with radiologically confirmed mLPLN, however, LPLNs is performed on lymph nodes suspected of harboring cancer instead. There have been limited reports of the oncologic effect of LPLNs, or “cherry picking” of nodes. However, recent research reported that LPLNs is not sufficient to get local control for rectal cancer patients treated with nCRT. In a large multicenter trial, Ogura et al. [11] compared results of patients who underwent formal LPLND and LPLNs, and 12 patients who received sampling of a mean of 2 sidewall nodes showed 51% of LR rated, compared with 5% in patients who received LPLND. Results of this study also showed that even patients who received LPLNs had worse outcomes than those who did not receive LPLNs. Based on the results of this study, we also agree that LPLNs might not have therapeutic benefits. Additionally, in our study, corresponding mLPLN is also difficult to identify with LPLNs, and in certain cases, no LPLN was extracted following LPLNs. Even in cases of minimally invasive surgery, there are considerable constraints because we must base all decisions on visual assessment alone. Thus, it is difficult to decide whether a lymph node is enlarged and to take relevant samples.
This study has several limitations. First, it was of retrospective and nonrandomized design; therefore, there is a possibility of selection bias and some data were absent from the medical records. Second, we did not remeasure LPLN SA because we believe it is an artificial measurement that has no significant impact on LPLN determination in real clinical practice. We concentrated on procedures conducted in actual clinical practice in this study. Finally, the number of patients who underwent LPLNs compared with the number enrolled in the study was small. Indeed, about 6% of patients underwent LPLNs, compared with 12% in earlier multicenter studies [11].
However, the advantages of this study are as follows: strengths of this study as are follows: first, it was a sizable cohort from a single center, and it accurately reflects clinically real practice in low rectal cancer. Second, the study had a relatively long follow-up; indeed, the median was approximately 60 months.
To summarize, we demonstrate that clinical mLPLN is associated with poorer DFS, OS, LRFS, and PRFS rates and that pre-nCRT and post-nCRT mLPLN (+) have different prognostic effects. When developing a treatment plan for rectal cancer patients, it is crucial to consider the impact of mLPLN at the initial stage on OS before undergoing nCRT. In addition, post-nCRT is primarily associated with DFS, specifically PR, and may require surgical intervention to enhance disease control.
Notes
References
1. Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995; 181:335–346. PMID: 7551328.
2. Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998; 133:894–899. PMID: 9711965.
3. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986; 1:1479–1482. PMID: 2425199.
4. Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999; 25:368–374. PMID: 10419706.
5. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001; 345:638–646. PMID: 11547717.
6. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–1740. PMID: 15496622.
7. Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006; 29:219–224. PMID: 16755173.
8. Ueno H, Mochizuki H, Hashiguchi Y, Hase K. Prognostic determinants of patients with lateral nodal involvement by rectal cancer. Ann Surg. 2001; 234:190–197. PMID: 11505064.
9. Ueno H, Yamauchi C, Hase K, Ichikura T, Mochizuki H. Clinicopathological study of intrapelvic cancer spread to the iliac area in lower rectal adenocarcinoma by serial sectioning. Br J Surg. 1999; 86:1532–1537. PMID: 10594501.
10. Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Miyoshi M, Kajiwara Y, et al. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. 2007; 245:80–87. PMID: 17197969.
11. Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, et al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. 2019; 37:33–43. PMID: 30403572.
12. Kim TG, Park W, Choi DH, Park HC, Kim SH, Cho YB, et al. Factors associated with lateral pelvic recurrence after curative resection following neoadjuvant chemoradiotherapy in rectal cancer patients. Int J Colorectal Dis. 2014; 29:193–200. PMID: 24322736.
13. Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJ, et al. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol. 2015; 111:459–464. PMID: 25559888.
14. Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008; 15:729–737. PMID: 18057989.
15. Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009; 10:1053–1062. PMID: 19767239.
16. Malakorn S, Chang GJ. Treatment of rectal cancer in the East and West: should it be different? Surgery. 2017; 162:315–316. PMID: 28619666.
17. Sammour T, Chang GJ. Lateral pelvic lymph node dissection and radiation treatment for rectal cancer: mutually exclusive or mutually beneficial? Ann Gastroenterol Surg. 2018; 2:348–350. PMID: 30238075.
18. Sammour T, Chang GJ. Lateral node dissection in low rectal cancer: time for a global approach? Ann Surg. 2017; 266:208–209. PMID: 28437315.
19. Malakorn S, Yang Y, Bednarski BK, Kaur H, You YN, Holliday EB, et al. Who should get lateral pelvic lymph node dissection after neoadjuvant chemoradiation? Dis Colon Rectum. 2019; 62:1158–1166. PMID: 31490825.
20. Otero de Pablos J, Mayol J. Controversies in the management of lateral pelvic lymph nodes in patients with advanced rectal cancer: East or West? Front Surg. 2020; 6:79. PMID: 32010707.
21. Garcia-Aguilar J, Cromwell JW, Marra C, Lee SH, Madoff RD, Rothenberger DA. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001; 44:1743–1748. PMID: 11742153.
22. Lee KH, Kim JS, Kim JY. Long-term oncologic outcomes of neoadjuvant concurrent chemoradiotherapy with capecitabine and radical surgery in locally advanced rectal cancer: 10-year experiences at a single institution. Ann Surg Treat Res. 2016; 91:178–186. PMID: 27757395.
23. Hojo K, Koyama Y, Moriya Y. Lymphatic spread and its prognostic value in patients with rectal cancer. Am J Surg. 1982; 144:350–354. PMID: 7114377.
24. Morikawa E, Yasutomi M, Shindou K, Matsuda T, Mori N, Hida J, et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon Rectum. 1994; 37:219–223. PMID: 8137667.
25. Kim YI, Jang JK, Park IJ, Park SH, Kim JB, Park JH, et al. Lateral lymph node and its association with distant recurrence in rectal cancer: a clue of systemic disease. Surg Oncol. 2020; 35:174–181. PMID: 32889250.
26. Zhou S, Jiang Y, Pei W, Liang J, Zhou Z. Prognostic significance of lateral pelvic lymph node dissection for middle-low rectal cancer patients with lateral pelvic lymph node metastasis: a propensity score matching study. BMC Cancer. 2022; 22:136. PMID: 35109810.
27. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004; 52:78–83. PMID: 15380850.
28. Lee D, Matsuda T, Yamashita K, Hasegawa H, Yamamoto M, Kanaji S, et al. Significance of lateral pelvic lymph node size in predicting metastasis and prognosis in rectal cancer. Anticancer Res. 2019; 39:993–998. PMID: 30711986.
29. Fujita S, Akasu T, Mizusawa J, Saito N, Kinugasa Y, Kanemitsu Y, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012; 13:616–621. PMID: 22591948.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–5 and Supplementary Fig. 1 can be found via https://doi.org/10.4174/astr.2023.104.4.205.
Supplementary Table 1
Clinicopathological features according to pre- and post-nCRT mLPLN
Supplementary Table 2
Univariable analysis of risk factors for overall survival and disease-free survival
Supplementary Table 4
Risk factors associated with pelvic recurrence-free survival
Supplementary Table 5
Risk factors associated with distant metastasis-free survival
Supplementary Fig. 1
Kaplan-Mayer analyses of the effects of LPLN sampling in pre-nCRT mLPLN (+) group (A, C, E, G) and post-nCRT mLPLN (+) group (B, D, F, H). Comparisons by logrank tests. nCRT, neoadjuvant chemoradiotherapy; mLPLN, lateral pelvic lymph node metastasis; LPLN, lateral pelvic lymph node.
Fig. 1
CONSORT (Consolidated Standards of Reporting Trials) diagram. nCRT, neoadjuvant chemoradiotherapy.
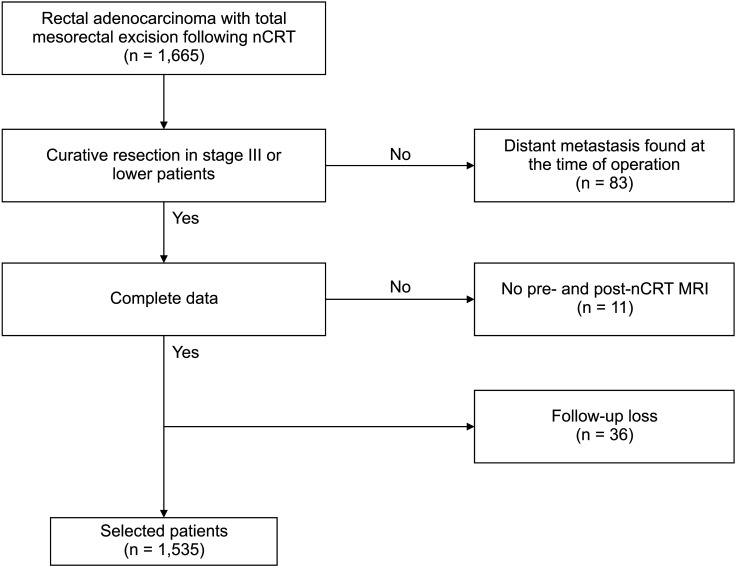
Fig. 2
Imaging study of a patient with an enlarged right internal iliac lymph node which is suspected as metastasized node. Pre-neoadjuvant chemoradiotherapy MRI T1-weighted image showing an enlarged right internal iliac lymph node with heterogenous signal (arrow).
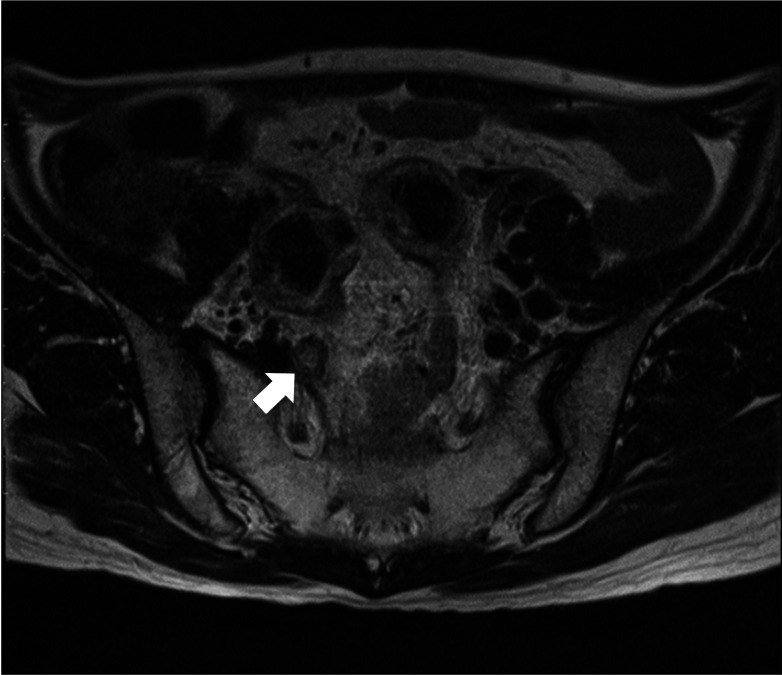
Fig. 3
Kaplan-Mayer analyses of the effects of pre-nCRT mLPLN on (A) disease-free survival (DFS), (B) overall survival (OS), (C) local recurrence-free survival (LRFS), and (D) pelvic recurrence-free survival (PRFS). Comparisons by log-rank tests. nCRT, neoadjuvant chemoradiotherapy; mLPLN, lateral pelvic lymph node metastasis.
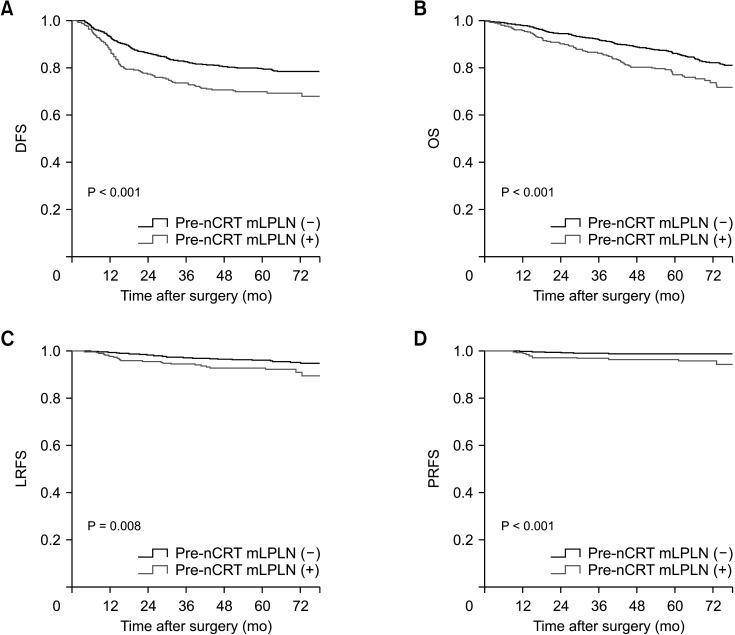
Fig. 4
Kaplan-Mayer analyses of the effects of post-nCRT mLPLN on (A) disease-free survival (DFS), (B) overall survival (OS), (C) local recurrence-free survival (LRFS), and (D) pelvic recurrence-free survival (PRFS). Comparisons by log-rank tests. nCRT, neoadjuvant chemoradiotherapy; mLPLN, lateral pelvic lymph node metastasis.
Table 3
Multivariable analysis of risk factors for overall survival and disease-free survival
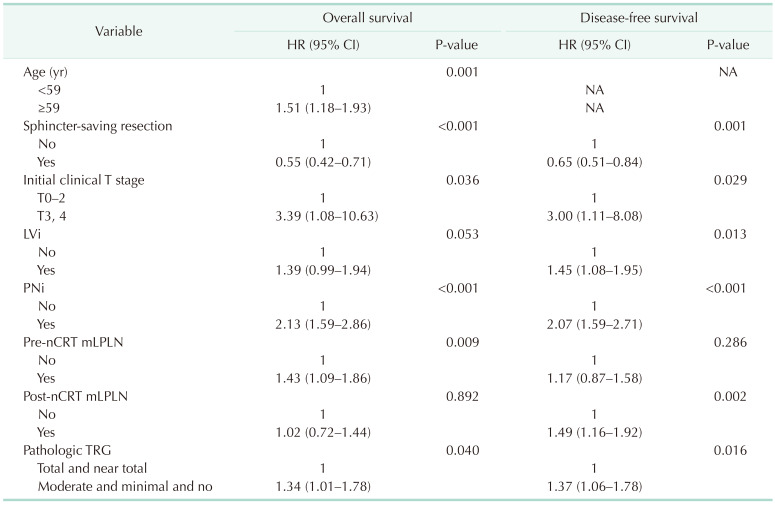
Factors significant on univariable analysis (Supplementary Table 1) were included in multivariable analysis.
HR, hazard ratio; CI, confidence interval; LVi, lymphovascualr invasion; PNi, perineural invasion; nCRT, neoadjuvant chemoradiotherapy; mLPLN, lateral pelvic lymph node metastasis; TRG, tumor regression grade.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download