Since omicron variant (B.1.1.529) became the fifth variant of concern (VOC) on November 26, 2021, omicron sublineages have led the coronavirus disease 2019 (COVID-19) outbreaks to date.
123 The diversity of circulating omicron sublineages, so called ‘variant soup,’ makes it more difficult to expect outbreak situation and potential effect of monoclonal antibody agents than before.
4 Unlike previous outbreak waves that were dominated by one or two dominant circulating variants,
15 the seventh domestic outbreak wave of Korea, which began on late October 2022 and ended on February 2023, showed a transition of dominant circulating variant from BA.5 to BN.1 with a mixture of other omicron sublineages. BA.5, BA.2.75, and XBB variants were derived from BA.2, and CH.1, CJ.1, and BN.1 were included in the sublineage of BA.2.75.
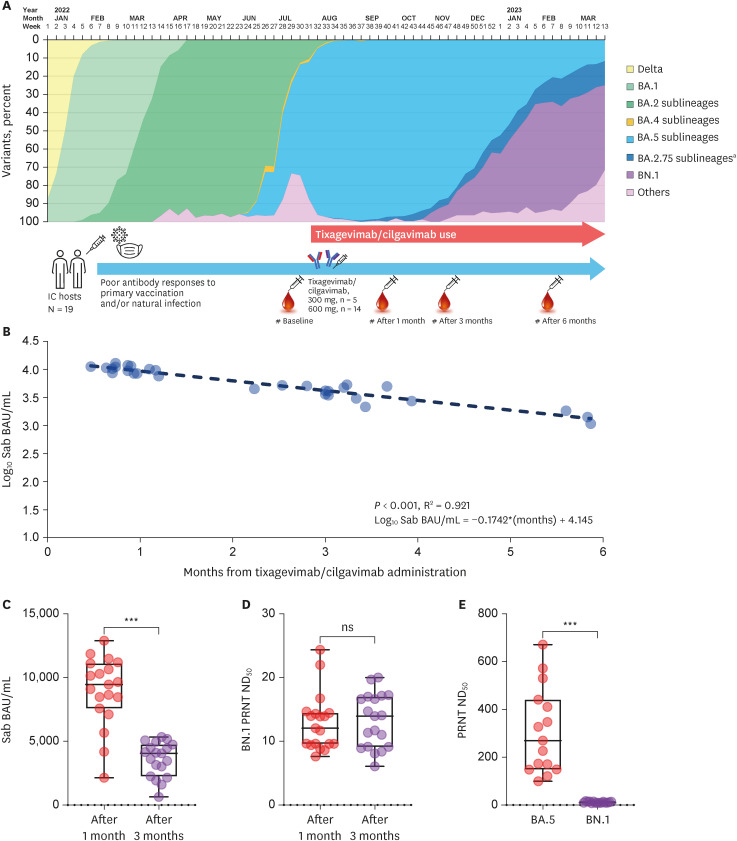
Fig. 1A presents the distribution of omicron sublineages in Korea from early 2022 to March 2023, along with sampling points of the study population. Tixagevimab/cilgavimab (Evusheld™; AstraZeneca, London, UK), a monoclonal antibody agent used to prevent COVID-19 among immunocompromised (IC) patients, was introduced in Korea on August, 2022, during the sixth domestic outbreak wave dominated by BA.5.
1 In previous publication, we demonstrated neutralizing activity of tixagevimab/cilgavimab against BA.5 comparable with healthy vaccinated individuals who experienced BA.1/BA.2 breakthrough infections, providing background evidence of tixagevimab/cilgavimab use during BA.5 dominance.
1 However, further experimental data about tixagevimab/cilgavimab activity against newly circulating strains, especially against BN.1, is required for its continuous use. Although the U.S. Food and Drug Administration-approved fact sheet of tixagevimab/cilgavimab suggested a moderately reduced activity against BN.1 (68-fold reduction), similar to that against BA.5 (33- to 65-fold reduction), but these data were based on the experiment of antibody-dependent viral entry into FcγRII-expressing Raji cells using recombinant virus-like particles (VLPs) pseudotyped with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike proteins.
6 To provide essential data whether to continue tixagevimab/cilgavimab use in Korea during BN.1 predominating period, we conducted the plaque reduction neutralization test (PRNT) against BN.1 and reviewed currently circulating strains and published data.
 | Fig. 1
Schematic view of the study and antibody kinetics after administration of tixagevimab/cilgavimab 600 mg. (A) Schematic view of the study, presented along with SARS-CoV-2 variant distribution in Korea from January 2022 to March 2023. We revised and extended the period of the Fig. 1 in the previously published article.1 After administration of tixagevimab/cilgavimab 600 mg, log10 Sab BAU/mL values exhibited a linearly waning kinetics (B). Compared to the peak point measured one month after admininistartion, a significant reduction of Sab titers was noticed after three months (C). PRNT ND50 titers against BN.1 were low at both points without significant difference, most of which were under the positive cut-off value of 20 (D). When the values of the peak point were compared, PRNT ND50 against BN.1 were significantly lower than that against BA.5 (E) unlike previously reported pseudotyped VLPs assay.6
IC = immunocompromised, Sab = anti-spike protein antibody, BAU = binding antibody unit, PRNT = plaque reduction neutralization test, ND50 = 50% neutralizing dose, VLP = virus-like particles, ns = not significant.
aExcept for BN.1, the proportion of which is presented separately.
***P < 0.001.

|
A prospective cohort study was conducted in a tertiary care hospital in Korea. Current indications for tixagevimab/cilgavimab administration in Korea were those who have not been diagnosed to be SARS-CoV-2 positive within seven days, who are ≥ 12 years of age, weigh ≥ 40kg, and meet one of the following conditions: 1) those receiving immunosuppressive therapy or high-intensity chemotherapy, 2) those who have received hematopoietic stem cell transplant or chimeric antigen receptor T cell therapy within six months, 3) those who have received a solid organ transplant within one year, 4) those who have human immunodeficiency virus infection and are not expected to have CD
4 T cell ≥ 200/mm
2 with sufficient treatment, and 5) those who have primary immunodeficiency. A total of 19 IC patients who met the above criteria were prospectively recruited, and received either 300 mg (n = 5) or 600 mg (n = 14) of tixagevimab/cilgavimab, based on the patient’s discretion (the same cohort participated in the previous publication
1). Blood sampling was conducted before and one, three, six months after tixagevimab/cilgavimab administration. During the follow-up period, specimens collected after administration of additional dose of tixagevimab/cilgavimab, intravenous immune globulin (IVIG), COVID-19 vaccination, or diagnosis of breakthrough infections were excluded from the present analysis.
The laboratory procedures were provided in the previous publication.
1 In brief, an electrochemiluminescence immunoassay kit (Elecsys Anti-SARS-CoV-2 S; Roche diagnostics, Basel, Switzerland) was used for the semi-quantitative measurement of anti-SARS-CoV-2 spike protein antibody (Sab). BN.1 sublineage (NCCP No. 43439) was utilized for BN.1 PRNT. To compare laboratory test results, Student’s
t-test was used. A linear regression model was utilized to assess the correlations between antibody titers and timeline. For the interpretation of the correlation coefficient, R
2 ≥ 0.7 was considered a strong correlation, R
2 ≥ 0.4 a moderate correlation, and R
2 < 0.4 a weak Correlation.
7 All
P values were two-sided tests, and those < 0.05 were considered statistically significant. GraphPad Prism version 9.4.1 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses.
Baseline characteristics of 19 IC patients were previously presented.
1 In brief, average age was 53.9 ± 13.8 years old and 13 (68.4%) were male. Nine (47.4%) patients had B-cell malignancy, nine (47.4%) received solid organ transplantation, and one (5.3%) had Good’s syndrome. Thirteen (68.4%) patients received at least two doses of vaccination and 10 (52.6%) patients had a previous history of SARS-CoV-2 infection. The Sab level at baseline sampling (624.8 ± 975.8 BAU/mL) was lower than the range of the 3rd vaccine response of a healthy adult cohort (range, 3,719.0–38,544.0 BAU/mL), and six patients (31.6%) showed negative result.
1
Fig. 1B shows a waning kinetics of 14 IC patients who received 600 mg of tixagevimab/cilgavimab. A total of 30 specimens were evaluated and log
10 Sab BAU/mL linearly decreases over time after tixagevimab/cilgavimab with a strong correlation (R
2 = 0.921,
P < 0.001). Based on a correlation equation deduced from the linear regression analysis (Log
10 Sab BAU/mL = −0.1742*[months] + 4.145), the peak Sab (10,136 ± 1,513 BAU/mL) measured one month after administration decreased to a half level approximately 3.5 months after administration. When the total 19 IC patients including five patients receiving 300 mg of tixagevimab/cilgavimab, the Sab level measured one month after administration (8,953 ± 2,715 BAU/mL) was decreased to less than half two months later (three months after administration, 3,602 ± 1,360 BAU/mL,
P < 0.001;
Fig. 1C). The same specimens were tested for BN.1 PRNT, and the average ND
50 were lower than the positive cut-off value at both one- and three-months after tixagevimab/cilgavimab administration (12.9 ± 4.5 and 13.2 ± 4.2, respectively,
P = 0.825;
Fig. 1D). When the specimens collected one month after administration were compared with BA.5 PRNT in pair (14 specimens), it was apparently noticed that the specimens could actively neutralize BA.5 (ND
50 310.5 ± 180.4), but not BN.1 (ND
50 11.5 ± 2.9,
P = 0.001;
Fig. 1E).
Since the beginning of COVID-19 pandemic, many anti-SARS-CoV-2 monoclonal antibody agents have been developed.
8910 Despite their clinical effectiveness, most of them were withdrawn from the market due to rapidly emerging SARS-CoV-2 variants. Also, short half-life of monoclonal antibody limited its usage only to the prevention of disease progression at the early phase of infection. Tixagevimab/cilgavimab was developed with two successful strategies to overcome these limitations. To overcome spike protein mutations of emerging variants, it was designed as a cocktail of two difference monoclonal antibodies that simultaneously bind to distinct non-overlapping epitopes of the viral spike protein.
11 An extended half-life was obtained by reengineering Fc variant amino acids, and therefore it could be used for pre-exposure prophylaxis in addition to early outpatient treatment, especially for poor vaccine responders such as organ transplant recipients or patients with hematologic malignancies.
111213141516 Despite mild to moderate decrease of antiviral activity against early omicron sublineages, including BA.1, BA.2, BA.4, and BA.5, it could be used during the omicron dominated outbreak period of 2022, by increasing dosage from 300 to 600 mg.
18 As it would not be feasible to perform neutralizing tests against all the newly emerging strains of live viruses, the manufacturer utilized VLP assay to reflect mutations of spike proteins.
6 However, unlike VLP assay result that exhibited moderately decreased activity to BN.1, similar with BA.5, tixagevimab/cilgavimab was not active against BN.1 in the present analysis. Such discrepancy was also noticed for BA.2.75 in the other publication.
17 Although BA.2.75 was reported to have moderately reduced susceptibility in the pseudotyped VLP assay, BA.2.75 was not susceptible in neutralizing assay.
17
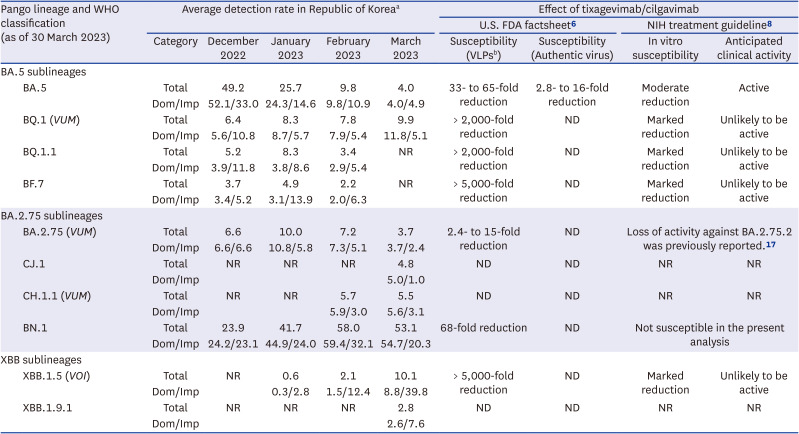
To interpret the present investigation in the context of domestic outbreak situation, we summarized detection rates of SARS-CoV-2 variants in Korea during the first quarter of 2023 and published data (
Table 1). Among the major circulating variants, BA.5 was the only strain known to be susceptible against tixagevimab/cilgavimab but the proportions rapidly decreased to 4.0% in March 2023. Sublineages of BA.2.75 are in increasing trend. BA.2.75 was reported to have moderately reduced susceptibility in the pseudotyped VLP assay, but was not susceptible in neutralizing assay in another report.
17 Likewise, BN.1 reported to have moderately reduced susceptibility in the pseudotyped VLP assay but was not susceptible in the present analysis. Taken together, the outbreak situation of the first quarter of 2023 is characterized with increasing trend of BA.2.75 sublineages, most of which show loss of susceptibility to tixagevimab/cilgavimab.
Table 1
Tixagevimab/cilgavimab susceptibility of SARS-CoV-2 variants circulating during the first quarter of 2023 in the Republic of Korea
|
Pango lineage and WHO classification (as of 30 March 2023) |
Average detection rate in Republic of Koreaa
|
Effect of tixagevimab/cilgavimab |
|
U.S. FDA factsheet6
|
NIH treatment guideline8
|
|
Category |
December 2022 |
January 2023 |
February 2023 |
March 2023 |
Susceptibility (VLPsb) |
Susceptibility (Authentic virus) |
In vitro susceptibility |
Anticipated clinical activity |
|
BA.5 sublineages |
|
|
|
|
|
|
|
|
|
|
BA.5 |
Total |
49.2 |
25.7 |
9.8 |
4.0 |
33- to 65-fold reduction |
2.8- to 16-fold reduction |
Moderate reduction |
Active |
|
Dom/Imp |
52.1/33.0 |
24.3/14.6 |
9.8/10.9 |
4.0/4.9 |
|
BQ.1 (VUM) |
Total |
6.4 |
8.3 |
7.8 |
9.9 |
> 2,000-fold reduction |
ND |
Marked reduction |
Unlikely to be active |
|
Dom/Imp |
5.6/10.8 |
8.7/5.7 |
7.9/5.4 |
11.8/5.1 |
|
BQ.1.1 |
Total |
5.2 |
8.3 |
3.4 |
NR |
> 2,000-fold reduction |
ND |
Marked reduction |
Unlikely to be active |
|
Dom/Imp |
3.9/11.8 |
3.8/8.6 |
2.9/5.4 |
|
BF.7 |
Total |
3.7 |
4.9 |
2.2 |
NR |
> 5,000-fold reduction |
ND |
Marked reduction |
Unlikely to be active |
|
Dom/Imp |
3.4/5.2 |
3.1/13.9 |
2.0/6.3 |
|
BA.2.75 sublineages |
|
|
|
|
|
|
|
|
|
|
BA.2.75 (VUM) |
Total |
6.6 |
10.0 |
7.2 |
3.7 |
2.4- to 15-fold reduction |
ND |
Loss of activity against BA.2.75.2 was previously reported.17
|
|
Dom/Imp |
6.6/6.6 |
10.8/5.8 |
7.3/5.1 |
3.7/2.4 |
|
CJ.1 |
Total |
NR |
NR |
NR |
4.8 |
ND |
ND |
NR |
NR |
|
Dom/Imp |
5.0/1.0 |
|
CH.1.1 (VUM) |
Total |
NR |
NR |
5.7 |
5.5 |
ND |
ND |
NR |
NR |
|
Dom/Imp |
5.9/3.0 |
5.6/3.1 |
|
BN.1 |
Total |
23.9 |
41.7 |
58.0 |
53.1 |
68-fold reduction |
ND |
Not susceptible in the present analysis |
|
Dom/Imp |
24.2/23.1 |
44.9/24.0 |
59.4/32.1 |
54.7/20.3 |
|
XBB sublineages |
|
|
|
|
|
|
|
|
|
|
XBB.1.5 (VOI) |
Total |
NR |
0.6 |
2.1 |
10.1 |
> 5,000-fold reduction |
ND |
Marked reduction |
Unlikely to be active |
|
Dom/Imp |
0.3/2.8 |
1.5/12.4 |
8.8/39.8 |
|
XBB.1.9.1 |
Total |
NR |
NR |
NR |
2.8 |
ND |
ND |
NR |
NR |
|
Dom/Imp |
2.6/7.6 |

The present study has several limitations. First, the number of enrolled patients was small and most of them were kidney transplant recipients and lymphoma patients. Nevertheless, we conducted a serial sampling up to six months after administration and exhibited a linear waning of Sab titers. The BN.1 PRNT was conducted for two time points and also compared with BA.5 PRNT. These results clearly presented loss of susceptibility of BN.1 to tixagevimab/cilgavimab and provides essential information on the practical decision whether to continue use of tixagevimab/cilgavimab. Second, PRNT against other circulating BA.2.75 sublineages, including CJ.1 and CH.1.1 was not conducted. Considering the same discrepant pattern between VLP assay and neutralizing test was noticed in BA.2.75 and BN.1, it would be less likely that these BA.2.75 sublineages would be susceptible to tixagevimab/cilgavimab. Tixagevimab/cilgavimab is no longer authorized in the United States as of January 26, 2023. Since it would not be effective against currently circulating SARS-CoV-2 variants, tixagevimab/cilgavimab should not be prescribed in current outbreak situation of Korea as well.
In conclusion, unlike VLP assay, this study using neutralizing test did not show activity of tixagevimab/cilgavimab against BN.1, the dominant circulating strain in Korea during the first quarter of 2023. Tixagevimab/cilgavimab would be less likely to be effective in the present predominance of BA.2.75 sublineages.
Ethics Statement
Informed consent was obtained from all the participants and this study was approved by Samsung Medical Center Institutional Review Board (SMC 2020-03-113 and 2021-11-050).
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download