2. Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002; 162(21):2443–2449. PMID:
12437403.
3. Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006; 114(13):1388–1394. PMID:
16982939.
4. Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007; 115(3):472–482. PMID:
17431501.
5. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021; 143(8):e254–e743. PMID:
33501848.
6. Jeong Y, Lee Y, Mun E, Seo E, Kim D, Lee J, et al. Overall and cardiovascular mortality according to 10-year cardiovascular risk of the general health checkup: the Kangbuk Samsung Cohort Study. Ann Occup Environ Med. 2022; 34(1):e40. PMID:
36544889.
7. Liu W, Zhang Y, Yu CM, Ji QW, Cai M, Zhao YX, et al. Current understanding of coronary artery calcification. J Geriatr Cardiol. 2015; 12(6):668–675. PMID:
26788045.
8. Venkataraman P, Stanton T, Liew D, Huynh Q, Nicholls SJ, Mitchell GK, et al. Coronary artery calcium scoring in cardiovascular risk assessment of people with family histories of early onset coronary artery disease. Med J Aust. 2020; 213(4):170–177. PMID:
32729135.
9. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018; 72(4):434–447. PMID:
30025580.
10. Rubin GD. Emerging and evolving roles for CT in screening for coronary heart disease. J Am Coll Radiol. 2013; 10(12):943–948. PMID:
24295945.
11. van der Bijl N, Joemai RM, Geleijns J, Bax JJ, Schuijf JD, de Roos A, et al. Assessment of Agatston coronary artery calcium score using contrast-enhanced CT coronary angiography. AJR Am J Roentgenol. 2010; 195(6):1299–1305. PMID:
21098187.
13. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15(4):827–832. PMID:
2407762.
14. McCollough CH, Ulzheimer S, Halliburton SS, Shanneik K, White RD, Kalender WA. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007; 243(2):527–538. PMID:
17456875.
15. Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA. 1994; 272(4):284–291. PMID:
8028141.
19. Eom SY, Lee YS, Lee SG, Seo MN, Choi BS, Kim YD, et al. Lead, mercury, and cadmium exposure in the Korean General Population. J Korean Med Sci. 2018; 33(2):e9. PMID:
29215818.
20. Surenbaatar U, Kim BG, Seo JW, Lim HJ, Kwon JY, Kang MK, et al. Environmental health survey for children residing near mining areas in South Gobi, Mongolia. Ann Occup Environ Med. 2021; 33(1):e10. PMID:
34754471.
21. Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006; 114(10):1538–1541. PMID:
17035139.
22. Kim S, Kang W, Cho S, Lim DY, Yoo Y, Park RJ, et al. Associations between blood lead levels and coronary artery stenosis measured using coronary computed tomography angiography. Environ Health Perspect. 2021; 129(2):27006. PMID:
33621129.
23. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340(2):115–126. PMID:
9887164.
24. Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int J Mol Sci. 2018; 19(6):1813. PMID:
29925772.
25. Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2008; 295(2):H454–H465. PMID:
18567711.
26. Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995; 25(2):155–161. PMID:
7843763.
27. Lee SY, Lee YJ, Min YS, Jang EC, Kwon SC, Lee I. The health effects of low blood lead level in oxidative stress as a marker, serum gamma-glutamyl transpeptidase level, in male steelworkers. Ann Occup Environ Med. 2022; 34(1):e34. PMID:
36544886.
28. Hertz-Picciotto I, Croft J. Review of the relation between blood lead and blood pressure. Epidemiol Rev. 1993; 15(2):352–373. PMID:
8174662.
29. Staessen JA, Bulpitt CJ, Fagard R, Lauwerys RR, Roels H, Thijs L, et al. Hypertension caused by low-level lead exposure: myth or fact? J Cardiovasc Risk. 1994; 1(1):87–97. PMID:
7614423.
30. Staessen JA, Roels H, Lauwerys RR, Amery A. Low-level lead exposure and blood pressure. J Hum Hypertens. 1995; 9(5):303–328. PMID:
7623368.
31. Schwartz J. Lead, blood pressure, and cardiovascular disease in men. Arch Environ Health. 1995; 50(1):31–37. PMID:
7717767.
32. Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens. 2002; 16(2):123–131. PMID:
11850770.
33. Ahn J, Kim NS, Lee BK, Park J, Kim Y. Association of blood pressure with blood lead and cadmium levels in Korean adolescents: analysis of data from the 2010-2016 Korean National Health and Nutrition Examination Survey. J Korean Med Sci. 2018; 33(44):e278. PMID:
30369859.
34. Harlan WR, Landis JR, Schmouder RL, Goldstein NG, Harlan LC. Blood lead and blood pressure. Relationship in the adolescent and adult US population. JAMA. 1985; 253(4):530–534. PMID:
3968785.
35. Hoffmann MH, Shi H, Schmitz BL, Schmid FT, Lieberknecht M, Schulze R, et al. Noninvasive coronary angiography with multislice computed tomography. JAMA. 2005; 293(20):2471–2478. PMID:
15914747.
36. Kuettner A, Beck T, Drosch T, Kettering K, Heuschmid M, Burgstahler C, et al. Diagnostic accuracy of noninvasive coronary imaging using 16-detector slice spiral computed tomography with 188 ms temporal resolution. J Am Coll Cardiol. 2005; 45(1):123–127. PMID:
15629385.
37. Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005; 26(15):1482–1487. PMID:
15840624.
38. Mollet NR, Cademartiri F, Krestin GP, McFadden EP, Arampatzis CA, Serruys PW, et al. Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol. 2005; 45(1):128–132. PMID:
15629386.
39. Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005; 46(3):552–557. PMID:
16053973.
40. Hahn D, Vogel N, Höra C, Kämpfe A, Schmied-Tobies M, Göen T, et al. The role of dietary factors on blood lead concentration in children and adolescents - Results from the nationally representative German Environmental Survey 2014-2017 (GerES V). Environ Pollut. 2022; 299:118699. PMID:
34929210.





 PDF
PDF Citation
Citation Print
Print



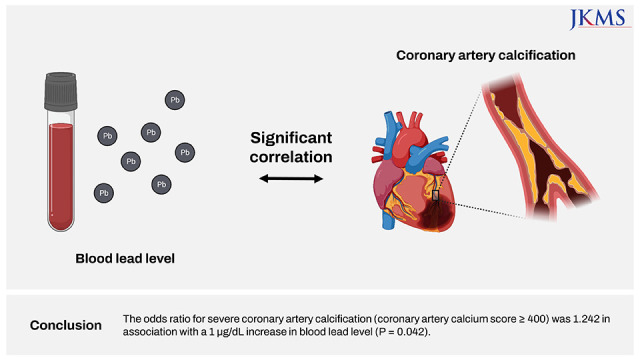
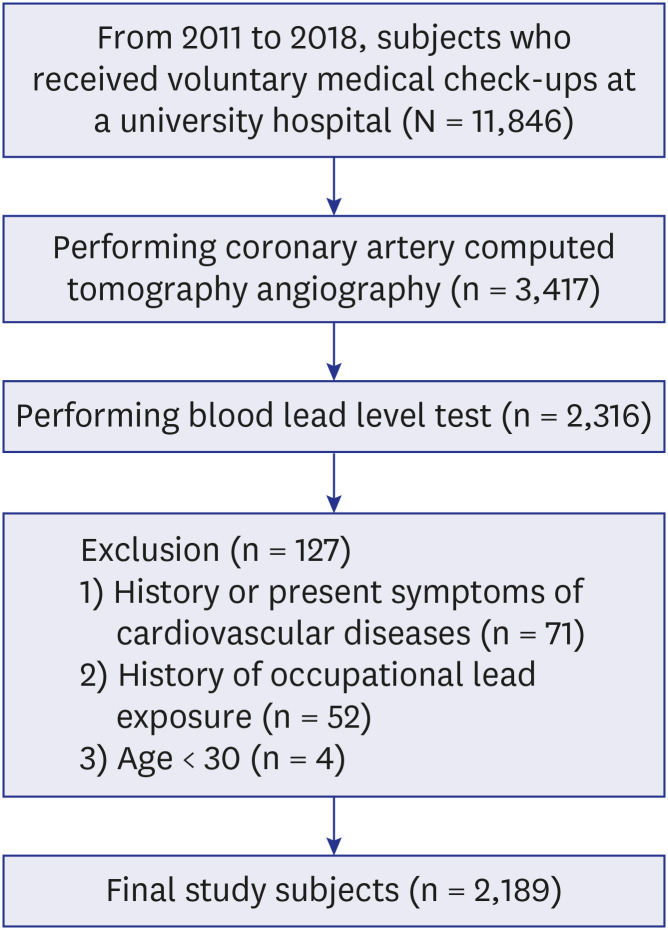
 XML Download
XML Download