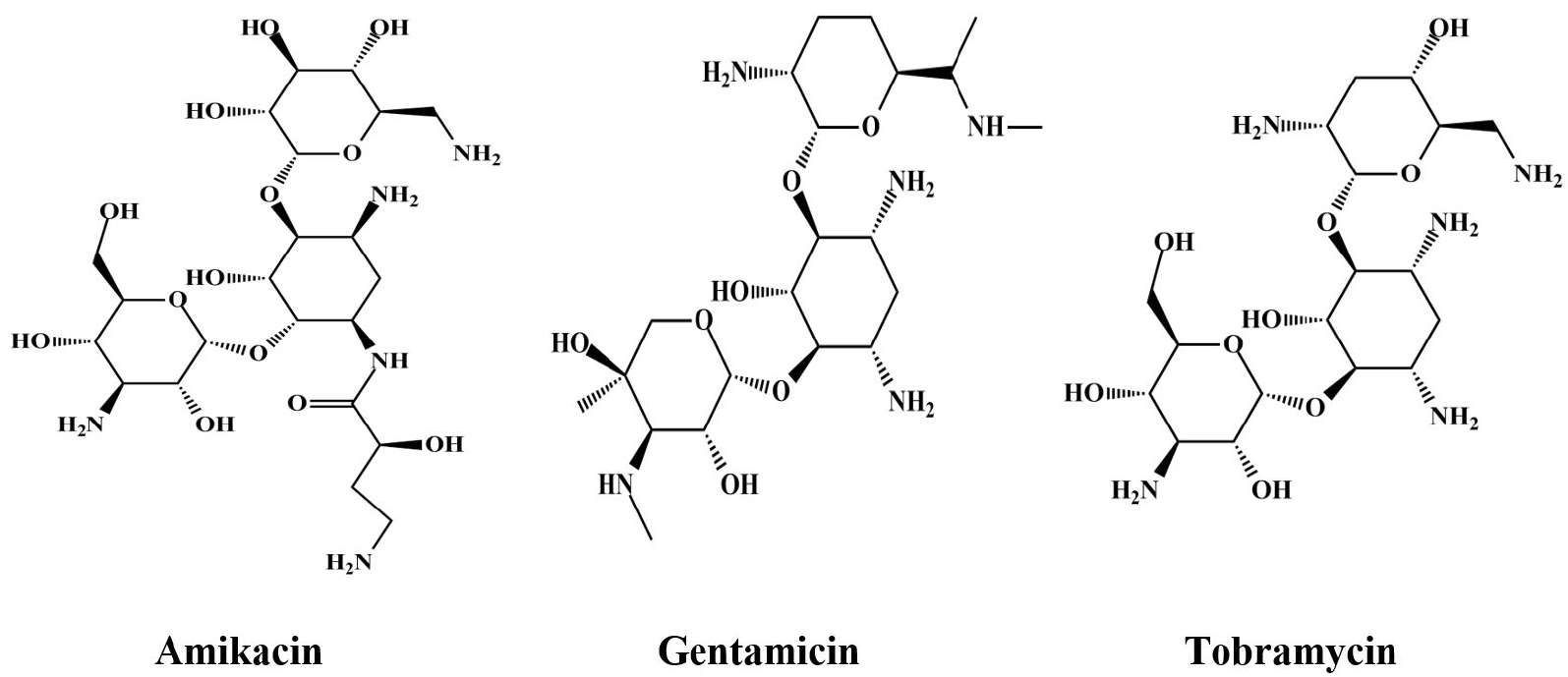
Commonly prescribed AGs (tobramycin, gentamicin, and amikacin) against gram-positive and -negative pathogens, including various types of AME and β-lactamase producing organisms, are shown in
Tables 1 and
2. The comparative minimum inhibitory concentration (MIC) values of plazomicin and other AGs against gram-negative bacteria (
Francisella tularensis and
Yersinia pestis, among others), common gram-negative aerobes and some anaerobes (
Prevotella spp. and Bacteroides only) are presented in
Table 1 (30,
31,
32,
33,
34,
35,
(36).
Table 2 (37) shows the effects of selected AMEs on the MIC of plazomicin and other traditional AGs. The susceptibility break point is ≤ 2 mg/L (FDA breakpoint) for plazomicin
(38). The equivalent activity of plazomicin was demonstrated against
Klebsiella pneumonia, non-ESBL producing
E. coli, and broad spectrum ESBL producing
E. coli with 90% of isolates suppressed by an MIC value of ≤ 1 mg/L
(38). Other AGs, such as tobramycin, gentamicin, and amikacin were more effective against
Pseudomonas aeruginosa and
Acinetobacter baumannii compared to plazomicin. Plazomicin is also less effective against these bacteria when its efficacy is compared against Enterobacteriaceae
(38). Plazomicin against carbapenem-resistant Enterobacteriaceae (CRE) exhibited MIC
90 and MIC
50 values of 1 and 0.5 mg/L in the U.S, respectively
(39). In contrast, it showed MIC
90 and MIC
50 values of 128 and 0.25 mg/L, in European and surrounding countries, respectively
(39). Plazomicin against carbapenem susceptible
A. baumannii (n = 8) and carbapenem resistant
A. baumannii (n = 69) showed MIC values ranging from 0.5–16 μg/mL and 0.5–64 μg/mL, respectively
(40). The AG inactivation by beta-lactam antibiotics has been observed
in vivo and
in vitro (41). Similar to other AGs, plazomicin showed lower
in vitro efficacy against
Stenotrophomonas maltophilia. It was evaluated in European countries against 99 isolates of
Acinetobacter spp. The evaluation showed an MIC
90 value of >128 mg/L with a susceptibility of 40.0%. Plazomicin demonstrated higher efficacy against Enterobacteriaceae than tobramycin or gentamicin. Although plazomicin is effective against these AMEs, which are the most described resistance mechanisms against AGs, it is not effective against less common 16S rRNA methyltransferases. However, plazomicin shows activity against Enterobacteriaceae which exhibits resistance mechanisms to other classes of antibiotics including metallo-beta-lactamases
(42). In an evaluation of plazomicin against ESBL-producing
K. pneumonia and
E. coli bacteria, colistin resistant Enterobacteriaceae, and CRE, it was more effective than other AGs and was comparable to ceftazidime/avibactam and vaborbactam/meropenem
(28,
31,
43,
44,
45). Plazomicin exhibits activity against gram-positive bacteria similar to that against gram-negative bacteria and works in a manner similar to that of other AGs
(31). The MIC
90 and MIC
50 range of plazomicin were 0.5–1 µg/mL and 0.25–0.5 µg/mL, respectively, against
Klebsiella, Escherichia, Enterobacter, Citrobacter, and
Serratia species
(11). Plazomicin was more effective against ESBL-producing Enterobacterales than were other AGs
(11). The MIC
90 of plazomicin was 1 mg/L against both methicillin susceptible
S. aureus and MRSA. It was also effective against
S. epidermidis with a MIC
90 value of 0.5 mg/L
(38). For plazomicin, the median MIC values of triplicate tests for different strains of
E. coli were 0.5, 1, 2, 2, and 4 mg/L for the AECO 1174, AECO 1175, AECO 1177, SMH 64979, and SMH 64982 strains, respectively
(46). The median MIC values of plazomicin for the different
K. pneumoniae strains were 0.5, 0.5, 1, 1, and 2 mg/L for the SMH 41965, SMH 41966, AKPN 1169, AKPN 1170, and AKPN 1171 strains, respectively
(46). The activity of this drug is confined to
Stenotrophomonas maltophilia,
A. baumannii, and
P. aeruginosa with majority of
in vitro data exhibiting MIC
50 of ≥ 4 mg/L with upper MIC allocations of plazomicin when compared to other AGs
(19).
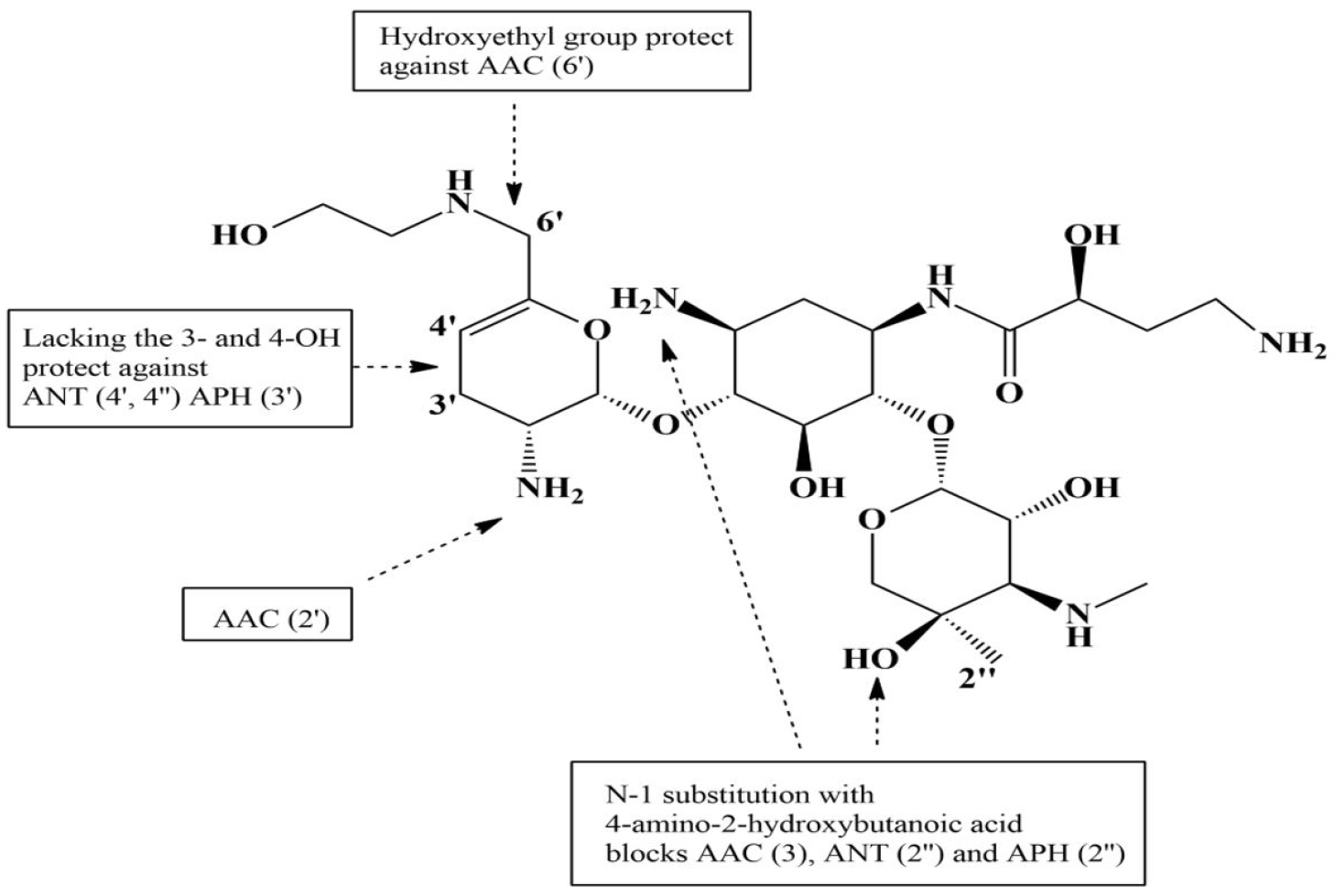




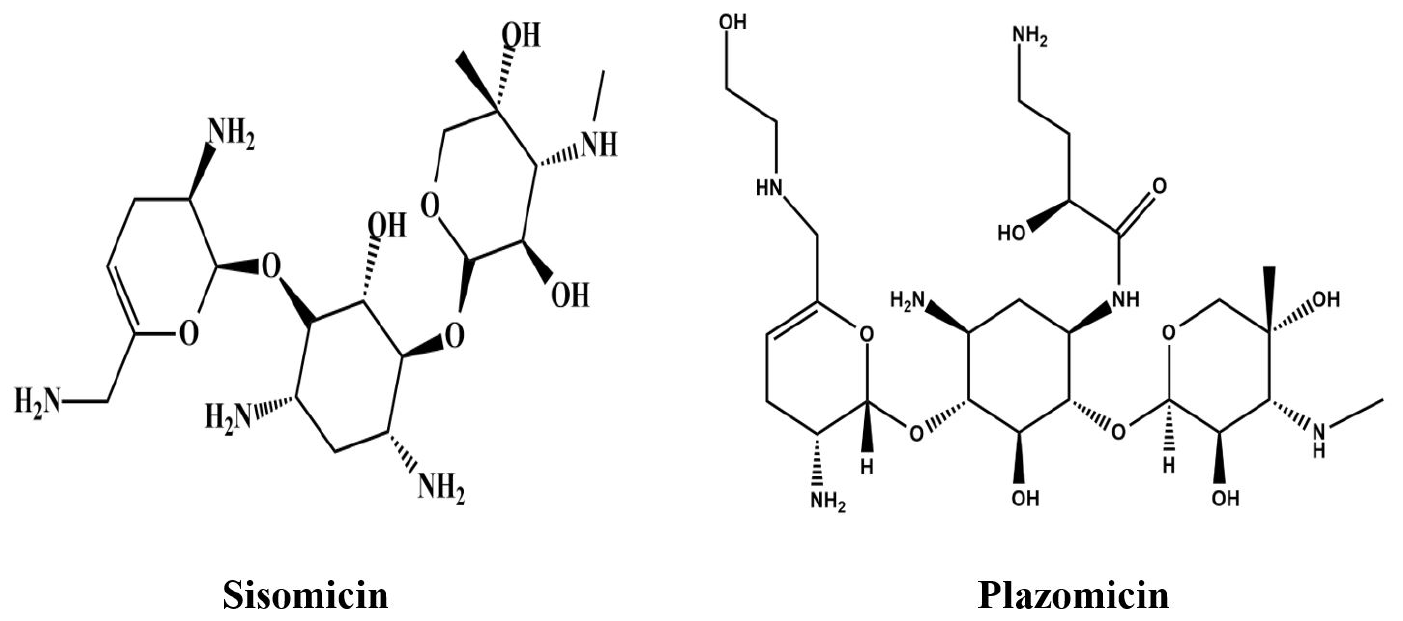
 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download