Abstract
Background
Case Report
Notes
Ethics statement
The local Ethics Committee of Asan Medical Center provided approval for this study (No. 2022-0357). The need for written informed consent was waived because this was a retrospective study.
Conflict of interest
Jun Young Chang is an editorial board member of the journal, but he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HR18C0016).
Author contributions
Conceptualization: all authors. Data curation: JCR, DL. Formal analysis: JCR. Funding acquisition: JYC. Investigation: JCR, JYC. Methodology: all authors. Project administration: JYC. Resources: all authors. Software: JCR, JYC. Supervision: SUK, JYC. Validation: JCR, JYC. Visualization: JCR. Writing–original draft: JCR, JYC. Writing–review & editing: all authors.
REFERENCES
Fig. 1.
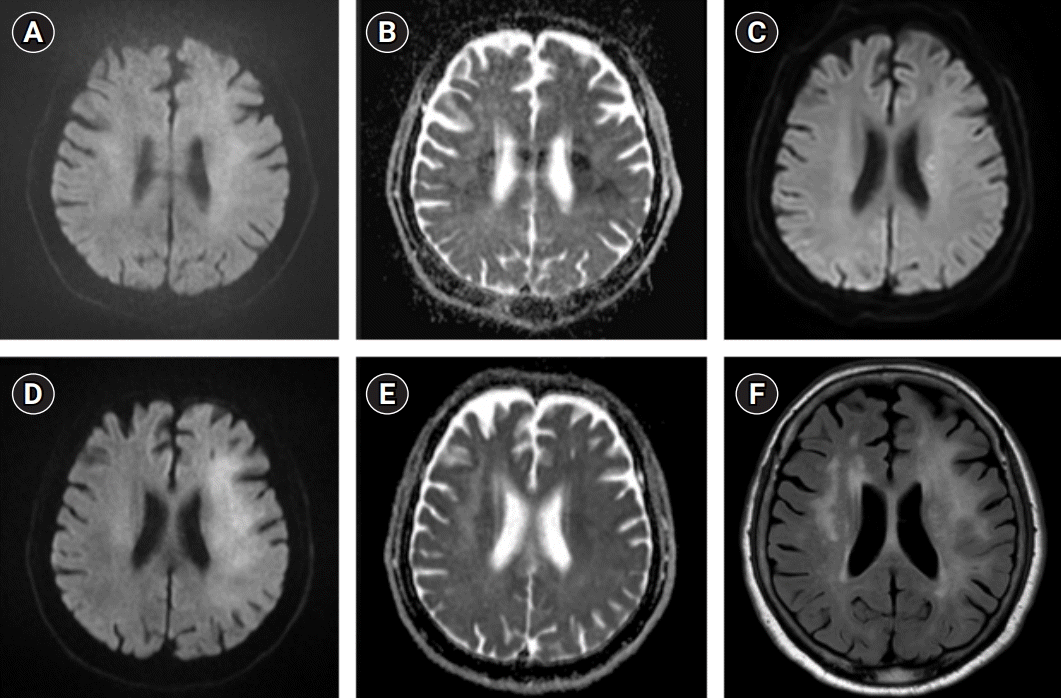
Fig. 2.
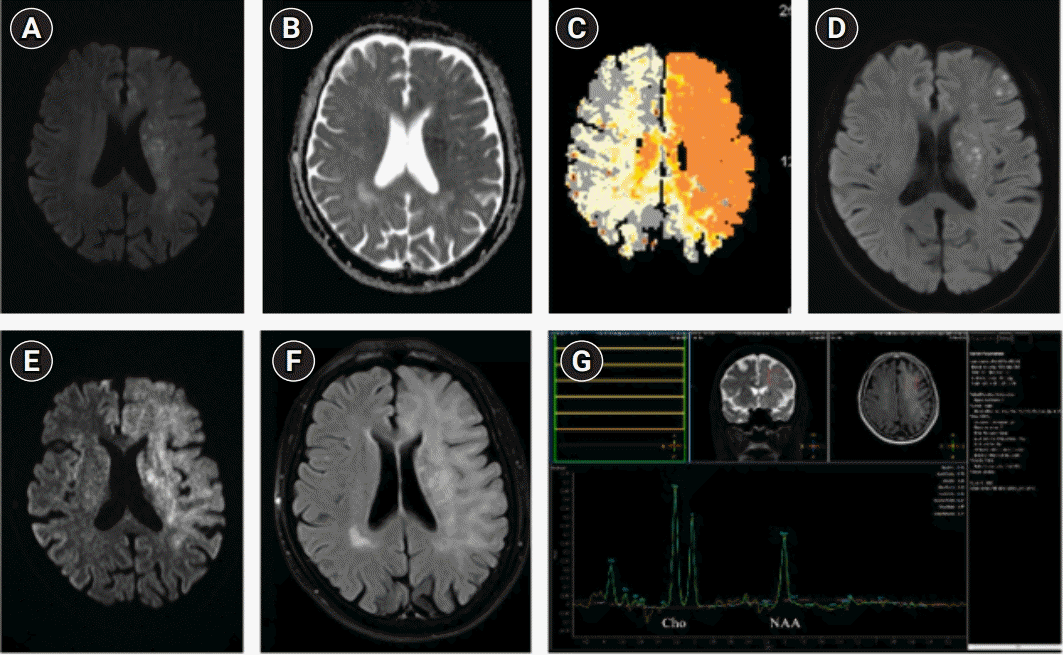
Fig. 3.
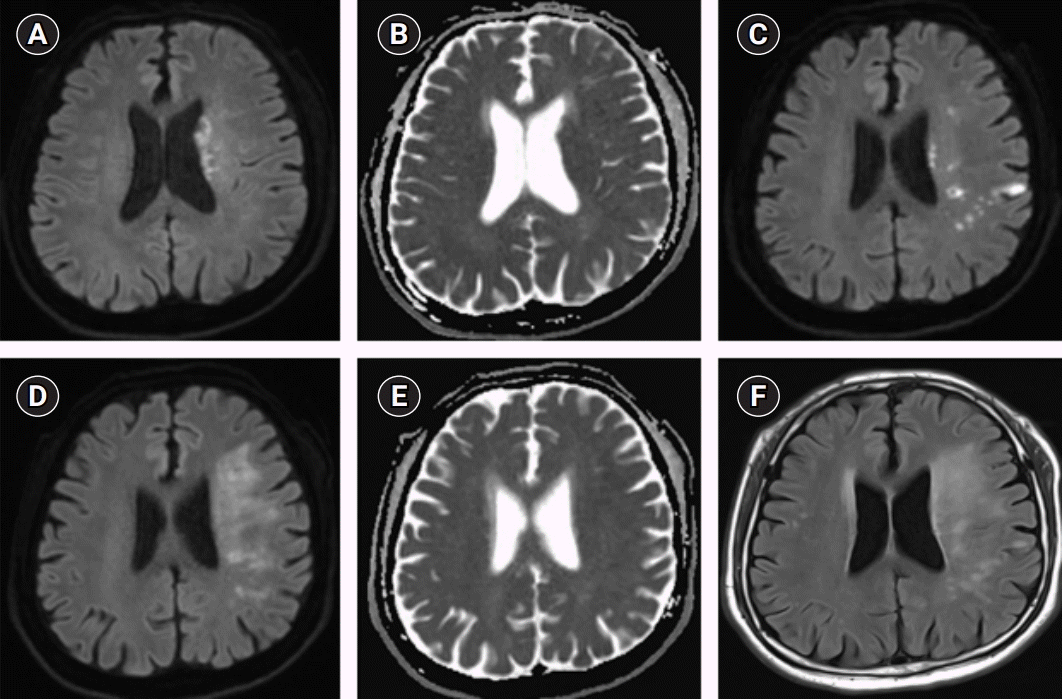
Table 1.
| Study | Sex/age (yr) | Risk factor | TOAST | Occlusion location | NIHSS (pre → post) | DWI ASPECTS | mTICI grade | HTf | Duration from stroke to delayed symptom (day) | Suspected trigger factor |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study (case 1) | F/79 | HT, AF, previous stroke | CE | Lt cavernous ICA | 21 → 6 | 9 | 3 | No | 28 | Fever, UTI, high BP |
| Current study (case 2) | F/77 | HT, HL, AF, CHF | CE | Lt cavernous ICA | 16 → 5 | 6 | 3 | HI-1 | 20 | None |
| Current study (case 3) | M/78 | HT, HL, AF, CAD, smoking | CE | Lt MCA M1 | 16 → 1 | 7 | 2b | No | 13 | None |
| Current study (case 4a)) | F/71 | HT, HL, AF, renal infarct | CE | Rt proximal CCA | 18 → 8 | 6 | 3 | HI-1 | 48 | High BP |
| Sasaki et al. (2017) [1] | F/79 | AF, CKD | CE | Rt MCA M1 | 9 → 0 | 10 | 3 | No | 70 | None |
| Singu et al. (2017) [2] | M/66 | HT, DM, HL, CAD, HF | CE | Lt MCA M1 | NA | NA | 2b or 3 | No | 35 | None |
| Nehme et al. (2019) [3] | F/71 | HT, DM, HL, CAD | CE | Lt terminal ICA | 18 → 2 | 10 (CT) | 3 | No | 18 | None |
TOAST, Trial of Org 10172 in Acute Stroke Treatment; NIHSS, National Institutes of Health Stroke Scale; DWI, diffusion-weighted imaging; ASPECTS, Alberta Stroke Program Early CT score; mTICI, modified thrombolysis in cerebral infarction; HTf, hemorrhagic transformation; HT, hypertension; AF, atrial fibrillation; CE, cardioembolism; Lt, left; ICA, internal carotid artery; UTI, urinary tract infection; BP, blood pressure; HL, hyperlipidemia; CHF, congestive heart failure; HI-1, hemorrhagic infarction type 1; CAD, coronary artery disease; MCA, middle cerebral artery; Rt, right; CCA, common carotid artery; CKD, chronic kidney disease; DM, diabetes mellitus; NA, not available; CT, computed tomography.
a)Not included in the current case reports.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download