Abstract
Background
Coronavirus disease 2019 (COVID-19) has resulted in social, economic, medical, and psychological changes. New-onset altered level of consciousness (ALC) is a classical presentation in real-world medicine. This study investigated changes in ALC in the emergency room (ER) in the periods before (BC) and after (AC) COVID-19.
Methods
This was a retrospective study of patients with ALCs who visited the ER of a tertiary referral center, and their medical records BC and AC were compared. A consortium allocated and analyzed the etiologies of ALC in a case-by-case discussion. The time point for etiological assessment was the time of discharge from the ER.
Results
In total, 1,936 patients with ALCs (731 and 1,205 in BC and AC, respectively) were investigated. The most common etiology was systemic infection (25.9%), followed by metabolic causes (20.8%). Systemic infections (22.9% vs. 30.8%, P<0.001) and stroke (14.6% vs. 18.2%, P=0.037) were lower in AC than in BC, respectively, whereas rates of toxicity (15.4% vs. 6.0%, P<0.001) and traumatic brain injury (TBI; 5.9% vs. 0.8%, P<0.001) were higher in AC than in BC. The overall mortality rate of ALC in the ER was 18.5%, which was higher in AC (20.9%) than in BC (14.6%) (P=0.001).
New-onset altered level of consciousness (ALC) refers to any non-physiological changes or deterioration in attention or arousal from baseline [1]. Since ALC is a potentially life-threatening condition [2], it has been a major consideration in the emergency room (ER). However, ALC has several synonyms, including mental change, altered mental status, and impaired consciousness, implying that ALC has a broad and nonspecific spectrum [2]. Furthermore, identifying or determining its etiology often needs to be clarified.
The prevalence of ALC in the ER has been reported in 0.4% to 5% of all patients visiting the ER; the common etiologies of ALC in the ER include neurologic causes [1,3,4], stroke [5], poisoning [6], pharmacologic and toxicologic etiologies [4], and systemic infection [7]. Recently, a multicenter study demonstrated that the leading etiology was metabolic causes, accounting for a quarter of the total cases, followed by systemic infection and stroke [8]. However, all studies to date have analyzed the data before the pandemic.
Coronavirus disease 2019 (COVID-19) has significantly impacted medical systems and personal lives. National health policies have changed because of the highly pathogenic severe acute respiratory syndrome coronavirus 2, and people's medical use patterns have also changed [9-11]. ER visits in the period after COVID-19 (AC), compared to those before COVID-19 (BC), decreased from 72% to 22% in the U.S. [12-15], 13% to 5.7% in Europe [16], and 50% to 37% in South Korea [17,18]. There has been a reduction in emergency medical service utilization, which was still under the pre-pandemic levels until the first quarter of 2022 [19].
Given the nature of ALC, which is a representative symptom of a critical condition, patients have no choice but to visit the ER. Medical staff should be aware of the changes in the etiology, and always be aware of the clinical characteristics, of ALC in the ER. This study aimed to evaluate the clinical characteristics, etiologies, and dispositions of patients with ALC in the ER of a university hospital and to compare BC and AC.
This retrospective study involved patients who visited the ER of a tertiary referral university hospital. In order to compare before and after the COVID-19 outbreak, we have set BC and AC as follows: BC represents the year before the first case occurred on February 18, from February 2019 to January 2020. After the first case of COVID-19 was found, there were frequent policy changes made by quarantine authorities, frequent ER closures, shortages of medical resources, and insufficient manpower. Perhaps every ER worldwide has suffered from the so-called coronapocalypse. Therefore, we excluded this disruptive period from our analysis. In this study, AC represents a year of new normalcy from July 2020 to June 2021 to represent the period after COVID-19 vaccination began in South Korea.
This study adopted the methods and inclusion criteria of the previous study [8]. Following suit, every case of ALC in the ER met one of the following conditions: (1) Glasgow Coma Scale (GCS) score ≤14, (2) impaired orientation (to person, time, and place), and (3) the first GCS examiner identified any findings considered ALC (e.g., bizarre or inappropriate behavior, hallucinations, delusions, or confusion). Each patient's GCS score was rated by either the attending board-certified faculty member or the chief resident in the Department of Emergency Medicine. The exclusion criteria were age <19 years, revisit within 24 hours of the last discharge from the hospital, cardiac arrest on arrival, death on arrival, and ALC that occurred during hospitalization.
Every patient with ALC in the ER was reviewed based on the medical records and allocated to one category of the 10-etiology classification system of ALC (ALC-10) [8]: (1) metabolic cause, (2) systemic infection, (3) cardiogenic and vascular cause (C&V), (4) stroke, (5) traumatic brain injury (TBI), (6) seizure, (7) central nervous system (CNS) infection, (8) toxic cause, (9) psychiatric disorder, and (10) undetermined. Patients’ age, sex, medical history, provisional diagnosis in the ER, destination from the ER, and discharge from hospital were investigated. Medical evaluation included vital signs, physical and neurological examinations, electrocardiography, echocardiography, X-rays, blood tests, computed tomography, magnetic resonance imaging, cerebrospinal fluid analysis, and electroencephalography. The reference time point for determining the etiology of ALC was when the patient left the ER; therefore, the provisional diagnosis may differ from the definitive diagnosis. The medical records of all patients were reviewed based on a multidisciplinary approach. A consortium of board-certified professors affiliated with emergency medicine, internal medicine, and neurology determined the etiology classification through case-by-case discussions.
In BC and AC, 39,273 and 35,968 patients visited the emergency department, respectively. We identified 1,936 eligible patients, including 731 and 1,205 patients with ALCs in BC and AC, respectively (Table 1, Fig. 1). More patients with ALC visited the ER in AC than in BC (3.4% vs. 1.9, P<0.001). There was no statistically significant difference in sex between BC (329, 45.0%) and AC (551, 45.7%) (female, P=0.758) (Table 1). The mean age was 68±17 years for all patients, and was higher in BC (67±17 years) than in AC (67±18 years) (P=0.023). The composition by age group did not differ between BC and AC (P=0.108). The most common age group was the 80s, and more than half of patients (1,056, 54.5%) were aged ≥70 years in this study, comprising 419 (57.3%) and 637 (52.9%) patients in BC and AC, respectively. The average stay time in the ER was 17.08±21.48 hours, with no significant difference between BC (17.89±22.11 hours) and AC (16.60±21.09 hours) (P=0.206).
Table 1 and Fig. 2 show the distribution of etiologies. The causes of ALC that accounted for more than 5% of the total cases included systemic infection (25.9%), metabolic causes (20.8%), stroke (16.0%), toxic causes (11.8%), C&V (7.1%), seizures (6.3%), and undetermined (5.3%). In both BC and AC, the most common etiology was systemic infection, although its proportion in BC (225, 30.8%) was significantly lower than that in AC (276, 22.9%) (P<0.001), followed by metabolic causes (21.1% and 20.6% for BC and AC, respectively). Stroke was the third most common in total cases (309, 16.0%) and in BC (133, 18.2%) but fourth in AC (176, 14.6%) (P=0.037). The rate of toxic causes differed remarkably between BC and AC (P<0.001). Only 44 (6.0%) patients in BC moved to the third position in AC (185, 15.4%). The number of TBIs was six in BC, accounting for 0.8%, and was significantly higher in AC (71, 5.9%) (P<0.001).
The three common extra-cranial etiologies, including systemic infection, metabolic causes, and toxic causes, accounted for 1,132 (58.5%) of the total cases, with 423 (57.9%) and 709 (58.8%) in BC and AC, respectively. The number of intracranial etiologies, including stroke, seizure, TBI, and CNS infection, was 528 (37.3% of the total), consisting of 196 (26.8%) and 332 (27.6%) in BC and AC, respectively.
Fig. 1 and Table 2 show the journey of the patients from disposition from the ER to discharge from the hospital. A total of 1116 (57.6%) of the patients with ALC in the ER were admitted to either the general ward (GW) (603, 54.0%) or the intensive care unit (ICU) (513, 46.0%). There was no difference between BC and AC in the proportions of patients admitted to the GW (P=0.327) and ICU (P=0.230). Patients discharged home from the ER showed no difference between BC and AC (P=0.757). A total of 299 patients were transferred to another hospital from the ER, which was lower in AC (169, 14.0%) than in BC (130, 17.8%) (P=0.027). Table 2 shows that 136 deaths occurred without admission or transfer, and mortality in the ER before disposition was higher in AC (106, 8.8%) than in BC (30, 4.1%) (P<0.001). After admission, the length of hospitalization of the patients with ALC in the ER was 19.25±25.19 days. It was longer in AC (20.63±27.74 days) than in BC (16.98±20.16 days) (P=0.012); however, there was no statistically significant difference in the destinations on discharge from the hospital.
Table 3 shows the mortality of ALC in the ER. A total of 369 patients died after visiting the ER, with an overall mortality rate of 18.5%. The mortality was higher in AC (252, 20.9%) than in BC (107, 14.6%) (P=0.001). The stroke mortality rates were 13.5% and 23.3% (P=0.031), whereas C&V mortality rates were 25.5% and 63.2% (P<0.001) in BC and AC, respectively.
ALC is a significant issue in the ER, and identification of its etiology, investigation of its clinical course, and preparation of a ready-made pathway are needed for real-world practice. This study provides important information by comparing the etiologies of ALC in BC and AC. ALC has many synonyms such as mental change, altered mental state, and loss of consciousness. It ranges from drowsiness to coma and includes confusion, psychosis, delusions, and abnormal behavior. This inconsistent nomenclature and broad spectrum of symptoms suggest that ALC can be transient and may be derived from diverse medical problems. In addition, the medical environment and medical utilization behaviors have changed owing to the pandemic, resulting in a change in the frequency of the etiologies.
When designing a study comparing BC and AC, we considered that the period of the coronapocalypse should be excluded. The COVID-19 outbreak and surge resulted in changes of national quarantine policy or the related suspension of emergency operations. Since there can be significant bias due to policy or political reasons rather than medical needs, we have stipulated that AC stands for the period after COVID-19 vaccination, to represent the so-called new normal.
There have been studies on changes in medical or ER utilization after the COVID-19 outbreak; however, there needs to be a more coherent definition of AC, discordant inclusion criteria, consistency in the study period, and etiology classification. Several studies have investigated periods of only 2 to 4 months to find a significant reduction in ER visits [15,20,21]. A few studies adopted a contrived definition of AC, with the number 2020 representing the year in the calendar rather than any medical gauge [18]. In addition, many disease- or condition-specific investigations have compared BC and AC, such as pneumonia [22], stroke [23,24], and suicide [25,26]. In contrast, few studies have evaluated ALC in the ER and compared BC and AC. An American study evaluated ALC in the ER in AC [21]; however, the study included only 166 patients over approximately 40 days, and its etiology classification was crude with only three categories: neurologic, metabolic, and indeterminate. Our study showed that the main etiologies of ALC in the ER were extra-cranial in AC and BC, and the leading cause was systemic infection. Intra-cranial etiologies accounted for approximately a quarter of the total. This finding is compatible with previous studies on BC [2,8,21]. Although the proportion of systemic infections decreased by approximately 8%, it was still the most common etiology of ALC in the ER, followed by metabolic causes. This implies that systemic infection and metabolic causes remain classic issues in the approach to the ALC in the ER.
The number of patients who visited the ER was higher in BC than in AC; however, the number of patients with ALC in AC was more than 1.5 times higher than that in BC. Between BC and AC, there were differences in the proportions of patients with toxic causes and stroke as ALC etiologies. Toxic causes include ingestion or inhalation of toxic materials such as alcohol, herbicides, caustic soda, and carbon monoxide, as well as medication overdose. According to the etiology classification system used in this study, any overdose was considered toxic. The remarkable increase in toxic cause can be explained by increased depression, suicidal ideation, and resultant suicidal attempts in AC [27,28]. Recent studies suggested that the incidence of stroke had decreased, and the patients were dispersed in AC [24,29,30]. In this study, the rating of the etiologies of ALC in the ER reflects the reality: more depression and suicides but fewer strokes in AC than in BC. In addition, this change resulted in a lower age in AC than in BC. The age of toxic causes was significantly lower than that of stroke (55.38±20.89 vs. 70.22±14.74 years, P<0.001). In previous studies, the mean age of patients with ALC visiting the ER varied from 65 to 69 years [2,5,8], which is consistent with the results of this study. The lower mean age in AC may be due to the remarkable difference in etiologies between BC and AC (more cases of toxic causes and TBIs in AC).
The data from this study alone cannot determine why the number of TBIs in AC was higher than that in BC. Recent studies have also reported an increase in TBIs [31,32]. However, the reason for this remains unclear. Based on our experience, frontline medical institutions lacked the manpower and facilities to handle ALC in TBI patients under the COVID-19 policy. Thus, ALC patients with TBI require a multidisciplinary approach and may need surgery. Therefore, in this university hospital, ALC with TBI was found to be increased in AC. Meanwhile, it is crucial that the etiology was undetermined in approximately 5% of ALC cases, despite 17.08±21.48 hours of stay time in the ER. There were several inevitable situations for which the cause of ALC was undetermined: the diagnosis often remained putative, two or more causes could not be excluded, some clinical situations led to an emergency operation, and some tests required several days to confirm the results (e.g., the aquaporin-4 antibody for neuromyelitis optica and cerebrospinal fluid cytopathology for carcinomatosis cerebri). This 5% rate implies that the clinical approach in the ER may be insufficient for the diagnosis and management of ALC, and additional medical approaches after hospitalization are required.
The average stay time in the ER did not differ between BC and AC in this study. A previous study reported that the ER stay time in AC increased in adult patients and decreased in pediatric patients [18]; however, the polymerase chain reaction test for COVID-19 takes about 6 hours, which was shorter than the average stay time to investigate ALC in the ER and resulted in no difference in stay time. Meanwhile, transfers from the ER to another hospital were reduced because of the quarantine policy in South Korea. Therefore, a negative polymerase chain reaction test for COVID-19 has been an essential prerequisite for all hospital admissions and transfers to another hospital. A previous study also reported an increase in hospitalization periods owing to COVID-19 testing [33]. According to this study, the quarantine policy is thought to have led to a decreased transfer rate from the ER, no change in the transfer rate upon discharge from the hospital, and an increase in the length of hospital stay in the ICU. The higher overall mortality rate and more deaths in the ER in AC were due to stroke and C&V that represent vascular attacks. There have been reports of an increase in the mortality rate of stroke or acute coronary syndrome related to COVID-19 [34,35], and our study supports these findings.
This study had several limitations. First, our study could not exclude selection bias because of the single-center retrospective design. Secondly, only a single ethnic background was considered. Third, the diagnoses in this study were provisional at the time of discharge from the ER, and may differ from the definite diagnosis. Despite these limitations, this is the first study to compare the etiology and mortality of ALC in the ER between AC and BC. The major etiologies of ALC in the ER were extra-cranial in both BC and AC, and the incidences of toxic cause as well as TBI were higher in AC. The mortality rate was higher in AC because of the higher mortality rates of mortality of systemic infection, stroke, and C&V. ALC is a significant issue in the ER because investigations can be time-consuming, multiple causes can coexist, and additional evaluation may be required beyond what the available time allows in the ER. These obstacles make the study of ALC in the ER a challenging but important area for further research.
Notes
Ethics statement
This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments and was approved by the Ethics Committee of Keimyung University Dongsan Medical Center (No. 2022-09-068). The requirement for written consent was waived because of the retrospective study design.
REFERENCES
1. Kanich W, Brady WJ, Huff JS, Perron AD, Holstege C, Lindbeck G, et al. Altered mental status: evaluation and etiology in the ED. Am J Emerg Med. 2002; 20:613–7.
2. Kim KT, Jeon JC, Jung CG, Park JA, Seo JG, Kwon DH. Etiologies of altered level of consciousness in the emergency room. Sci Rep. 2022; 12:4972.
3. Kekec Z, Senol V, Koc F, Seydaoglu G. Analysis of altered mental status in Turkey. Int J Neurosci. 2008; 118:609–17.
4. Xiao HY, Wang YX, Xu TD, Zhu HD, Guo SB, Wang Z, et al. Evaluation and treatment of altered mental status patients in the emergency department: life in the fast lane. World J Emerg Med. 2012; 3:270–7.
5. Völk S, Koedel U, Pfister HW, Schwankhart R, Op den Winkel M, Mühlbauer K, et al. Impaired consciousness in the emergency department. Eur Neurol. 2018; 80:179–86.
6. Forsberg S, Höjer J, Enander C, Ludwigs U. Coma and impaired consciousness in the emergency room: characteristics of poisoning versus other causes. Emerg Med J. 2009; 26:100–2.
7. Jung S, Jeon JC, Jung CG, Cho YW, Kim KT. The etiologies of altered level of consciousness in the emergency department. J Neurocrit Care. 2020; 13:86–92.
8. Kim KT, Kwon DH, Jeon JC, Kim IC, Park JA, Seo JG. A multicenter study of altered level of consciousness in the emergency room. Intern Emerg Med. 2022; 17:2329–37.
9. Ristau P, Wnent J, Gräsner JT, Fischer M, Bohn A, Bein B, et al. Impact of COVID-19 on out-of-hospital cardiac arrest: a registry-based cohort-study from the German Resuscitation Registry. PLoS One. 2022; 17:e0274314.
10. Lu M, Liao X. Access to care through telehealth among U.S. Medicare beneficiaries in the wake of the COVID-19 pandemic. Front Public Health. 2022; 10:946944.
11. Richards JR, Derlet RW. Emergency department hallway care from the millennium to the pandemic: a clear and present danger. J Emerg Med. 2022; 63:565–8.
12. Adjemian J, Hartnett KP, Kite-Powell A, DeVies J, Azondekon R, Radhakrishnan L, et al. Update: COVID-19 pandemic-associated changes in emergency department visits: United States, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:552–6.
13. Westgard BC, Morgan MW, Vazquez-Benitez G, Erickson LO, Zwank MD. An analysis of changes in emergency department visits after a state declaration during the time of COVID-19. Ann Emerg Med. 2020; 76:595–601.
14. Reschen ME, Bowen J, Novak A, Giles M, Singh S, Lasserson D, et al. Impact of the COVID-19 pandemic on emergency department attendances and acute medical admissions. BMC Emerg Med. 2021; 21:143.
15. Jeffery MM, D’Onofrio G, Paek H, Platts-Mills TF, Soares WE 3rd, Hoppe JA, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med. 2020; 180:1328–33.
16. Laukkanen L, Lahtinen S, Liisanantti J, Kaakinen T, Ehrola A, Raatiniemi L. Early impact of the COVID-19 pandemic and social restrictions on ambulance missions. Eur J Public Health. 2021; 31:1090–5.
17. Kim YS, Kim IB, Kim SR, Cho BJ. Changes in emergency department case severity and length of stay before and after COVID-19 outbreak in Korea. Healthcare (Basel). 2022; 10:1540.
18. Chang H, Kang MW, Paek SH. Impact of the COVID-19 pandemic on emergency department utilization patterns in South Korea: a retrospective observational study. Medicine (Baltimore). 2022; 101:e29009.
19. Melnick G, O’Leary JF, Zaniello BA, Abrishamian L. COVID-19 driven decline in emergency visits: has it continued, is it permanent, and what does it mean for emergency physicians? Am J Emerg Med. 2022; 61:64–7.
20. Ghaderi H, Stowell JR, Akhter M, Norquist C, Pugsley P, Subbian V. Impact of COVID-19 pandemic on emergency department visits: a regional case study of informatics challenges and opportunities. AMIA Annu Symp Proc. 2022; 2021:496–505.
21. Antoniello D, Milstein MJ, Dardick J, Fernandez-Torres J, Lu J, Patel N, et al. Altered mental status in COVID-19. J Neurol. 2022; 269:12–8.
22. Ha JY, Sung WY. Impact of COVID-19 pandemic on emergency department length of stay and clinical outcomes of patients with severe pneumonia: a single-center observational study. Medicine (Baltimore). 2022; 101:e30633.
23. Tani T, Imai S, Fushimi K. Impact of the COVID-19 pandemic on emergency admission for patients with stroke: a time series study in Japan. Neurol Res Pract. 2021; 3:64.
24. Park B, Bae W, Kim HJ, Lim JY, Oh SH, Youn CS, et al. Impact of COVID-19 pandemic on patients with cardio/cerebrovascular disease who visit the emergency department. Am J Emerg Med. 2022; 58:100–5.
25. Kang JH, Lee SW, Ji JG, Yu JK, Jang YD, Kim SJ, et al. Changes in the pattern of suicide attempters visiting the emergency room after COVID-19 pandemic: an observational cross sectional study. BMC Psychiatry. 2021; 21:571.
26. Holland KM, Jones C, Vivolo-Kantor AM, Idaikkadar N, Zwald M, Hoots B, et al. Trends in US emergency department visits for mental health, overdose, and violence outcomes before and during the COVID-19 pandemic. JAMA Psychiatry. 2021; 78:372–9.
27. Farooq S, Tunmore J, Wajid Ali M, Ayub M. Suicide, self-harm and suicidal ideation during COVID-19: a systematic review. Psychiatry Res. 2021; 306:114228.
28. COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021; 398:1700–12.
29. Kim J, Kim C, Park SY. Impact of COVID-19 on emergency medical services for patients with acute stroke presentation in Busan, South Korea. J Clin Med. 2021; 11:94.
30. Sedova P, Kent JA, Bryndziar T, Jarkovsky J, Tomek A, Sramek M, et al. The decline in stroke hospitalization due to COVID-19 is unrelated to COVID-19 intensity. Eur J Neurol. 2023; 30:943–50.
31. Damara FA, Muchamad GR, Anton A, Ramdhani AN, Channel IC, Faried A. Epidemiological pattern of traumatic brain injury in the COVID-19 pandemic: a systematic review and meta-analysis. World Neurosurg. 2022; 161:e698–709.
32. Prawiroharjo P, Pangeran D, Supriawan H, Lastri D, Mayza A, Zairinal RA, et al. Increasing traumatic brain injury incidence during COVID-19 pandemic in the emergency department of Cipto Mangunkusumo National General Hospital: a national referral hospital in Indonesia. Neurology. 2020; 95(12 Suppl 2):S11.
33. Thai PQ, Toan DT, Son DT, Van HT, Minh LN, Hung LX, et al. Factors associated with the duration of hospitalisation among COVID-19 patients in Vietnam: a survival analysis. Epidemiol Infect. 2020; 148:e114.
34. Martí-Fàbregas J, Guisado-Alonso D, Delgado-Mederos R, Martínez-Domeño A, Prats-Sánchez L, Guasch-Jiménez M, et al. Impact of COVID-19 infection on the outcome of patients with ischemic stroke. Stroke. 2021; 52:3908–17.
35. Teixeira-Vaz A, Rocha JA, Costa A, Simões Moreira T, Almeida E Reis D, Oliveira M, et al. What is the impact of previous cerebrovascular disease on critical COVID-19 patients’ mortality?: a prospective cohort study. J Neurol Sci. 2022; 442:120382.
Fig. 1.
Flow diagram for disposition of patients with altered level of consciousness before (BC) and after (AC) coronavirus disease 2019. ER, emergency room; ALC, altered level of consciousness; GW, general ward; ICU, intensive care unit.
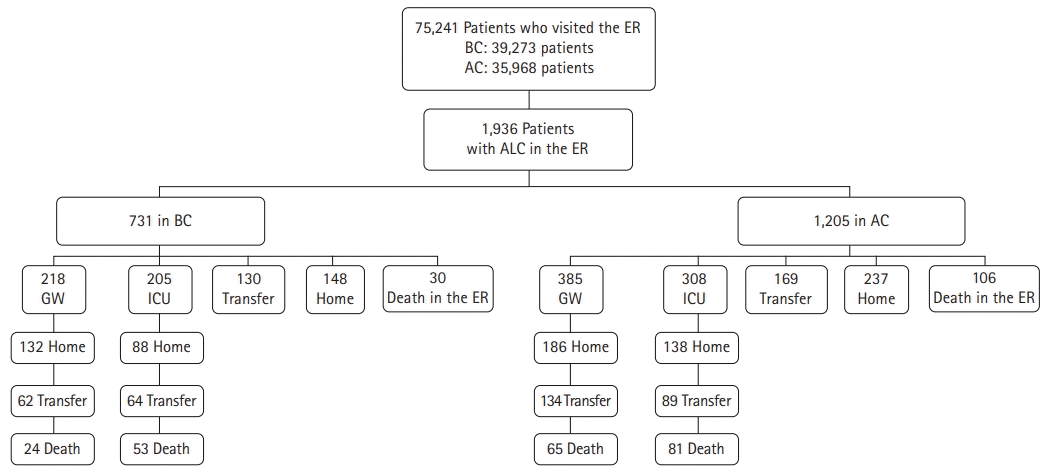
Fig. 2.
Pie graph for etiology of altered level of consciousness in patients (A) before and (B) after coronavirus disease 2019 (COVID-19). CNS, central nervous system; TBI, traumatic brain injury; C&V, cardiogenic and vascular cause.
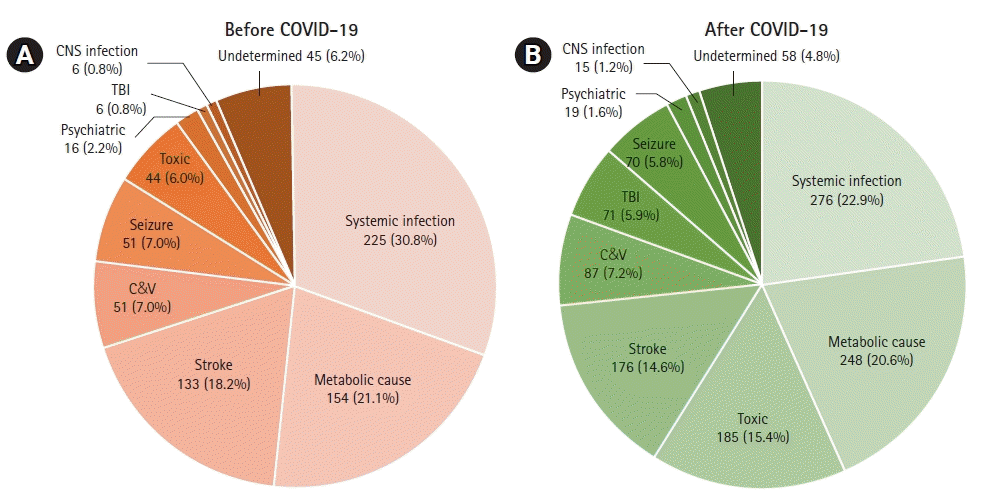
Table 1.
Demographic data and the etiologies of altered level of consciousness in the emergency room
Table 2.
Dispositions and destinations of patients with altered level of consciousness
Table 3.
Mortality by etiology and overall mortality




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download