Introduction
Abnormal urinalysis (AUA) in children, characterized by any abnormal levels of certain substances, cells, or even microorganisms in the urine, is one of the most common reasons that patients are referred to nephrologists. AUA can range from asymptomatic to macroscopic hematuria; these can be caused by transient conditions, such as urinary tract infections (UTIs) or underlying chronic diseases, such as glomerulonephritis (GN) [
1]. Since conditions that may cause AUA are diverse, their treatment and outcomes also vary. For example, the Alport syndrome does not respond to immunosuppressive management and invariably progresses to end-stage kidney disease, while lupus nephropathy requires prolonged immunosuppressants (IS), and postinfectious GN remits spontaneously. Moreover, benign conditions, such as nutcracker syndrome, which does not require management, may also present with AUA. Therefore, it is essential to obtain a precise diagnosis and manage the underlying condition that can be diagnosed through detailed history taking, including familial history, ultrasonography, blood and urine examination, and sometimes, kidney biopsy [
2-
5].
According to reports of school screening programs in East Asia, 0.01% to 5.10% of children had an AUA [
6,
7]. Isolated hematuria is the most common finding, comprising approximately two-thirds of the AUA cases [
6,
7]. Children with isolated hematuria are likely to have normal or benign etiology and good prognosis [
8,
9], whereas children with persistent combined hematuria and proteinuria are likely to have chronic GN [
7,
8,
10]. When chronic GN is suspected, prompt management is required because it may progress to end-stage kidney disease, if left untreated. However, the management of GN often includes IS, which has significant side effects. Therefore, a definitive diagnosis is crucial before starting potentially harmful treatments, such as IS. This is reflected in international and national guidelines. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines in 2021 recommend that kidney biopsies be performed in patients with persistent glomerular hematuria and/or proteinuria [
3]. A recently produced Korean guideline on hematuria in children also recommends kidney biopsies when persistent hematuria is accompanied by proteinuria, hypertension, or decreased kidney function [
2]. However, not all pediatric nephrologists perform kidney biopsies before initiating IS. To assess the clinical practice pattern of AUA in children, we surveyed the opinions of members of the Korean Society of Pediatric Nephrology (KSPN) using a clinical vignette. Herein, we report the results of the survey along with a discussion.
Go to :

Discussion
This survey revealed that the clinical practice patterns of AUA in children are diverse. Although about 5% of the email recipients responded to the survey, we assume that the survey captured the opinions of more than half of the active members of the KSPN, considering that the total number of active, certified pediatric nephrologists in Korea is approximately 40, and the responder’s experience and age are not concentrated. When comparing the responses of nephrologists and others, the most prominent difference was that most nephrologists chose to perform a kidney biopsy before starting any treatment, whereas the others were willing to perform a steroid pulse even without a kidney biopsy.
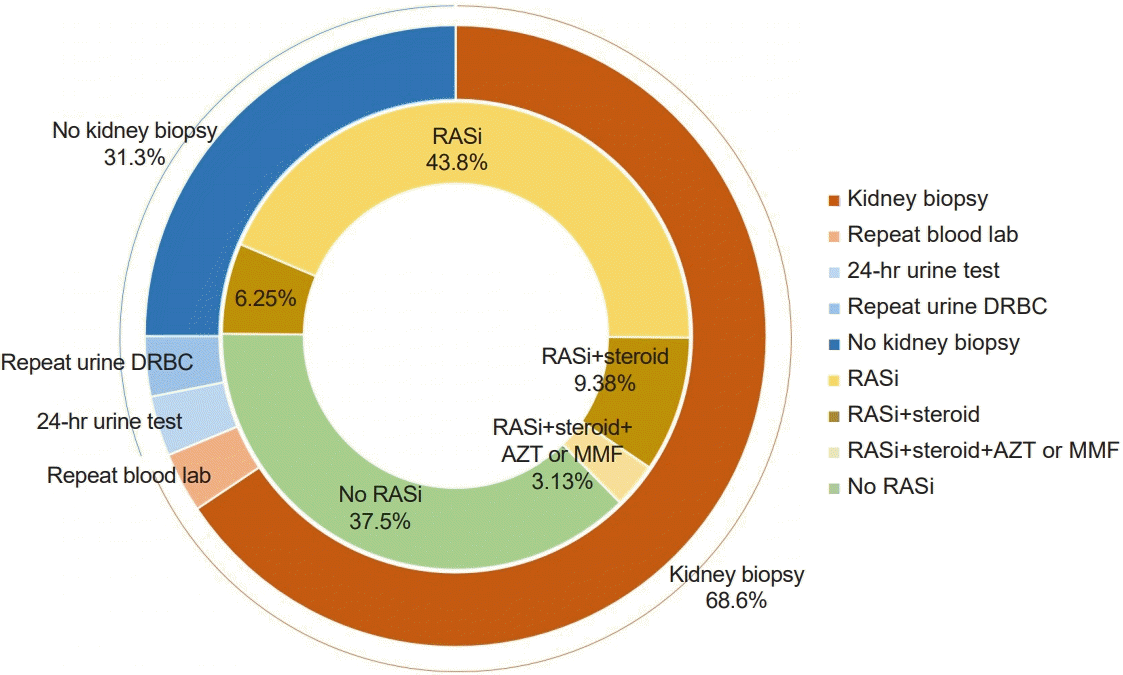
The initial part of the survey assessed practice patterns of the diagnostic approach. While most responders suspected GN, only two-thirds answered that they had performed kidney biopsies. However, kidney biopsy is essential for the diagnosis of GN since the clinical presentation itself is not different between various glomerulopathies, including genetic diseases. Kidney biopsy is an invasive procedure that may lead to complications, such as hematuria, hematoma, arteriovenous fistula, and even loss of the kidney [
11,
12]. In addition, approximately 15% of the children who undergo kidney biopsies may not obtain positive results [
13,
14]. Those were why clinicians are reluctant to kidney biopsies. Of course, it is possible to diagnose GN through biomarkers (phospholipase A2 receptor antibody in membranous nephropathy or myeloperoxidase in antineutrophil cytoplasmic antibody vasculitis) and genetic tests in the Alport disease or Fabry disease or by diagnosing systemic diseases, such as systemic lupus erythematosus [
3]. However, the clinical vignette of the survey was not compatible with any situation in which a biopsy could be exempted. Suspected cases of GN require a kidney biopsy as an accurate diagnosis could change treatment and prognosis [
15].
The treatment for hematuria is based on the underlying disease, not the hematuria itself. RAS inhibition is recommended as the first-line treatment when a child was diagnosed with GN, such as IgAN [
3], as our responders chose. In the same situation, six responders answered to start steroids. However, because IS are accompanied by immune suppression and complications, such as growth suppression, cushingoid features, and osteoporosis [
16], IS should only be used when indicated even after a definitive diagnosis. According to the KDIGO and Japanese guidelines for IgAN, even if a patient is diagnosed with IgAN, IS are recommended when patients are at a high risk of chronic kidney disease (CKD): severe proteinuria despite RAS blockage or mesangial hypercellularity in a kidney biopsy [
3,
17].
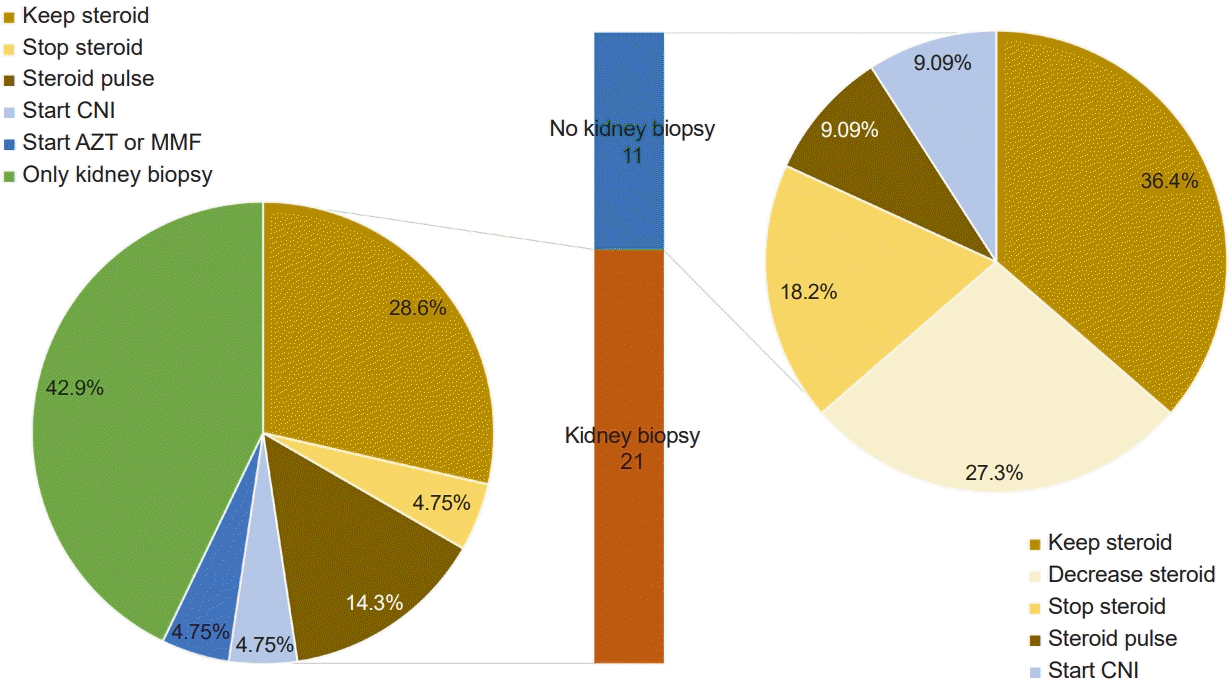
Similarly, steroid pulse therapy is considered when patients are indicated. The clinical vignette of the survey was managed with steroid pulse therapy when proteinuria persisted despite 5 weeks of oral steroids, and four responders agreed with this. However, methylprednisolone pulse therapy as early treatment is indicated only for steroid-resistant nephrotic syndrome and lupus nephritis. In IgAN, one study assessed the efficacy of steroid pulse therapy. Pozzi et al. [
18] reported that intravenous high-dose methylprednisolone (1 g/day for 3 consecutive days, every 2 months) followed by oral prednisolone for 6 months was effective in decreasing proteinuria, but the indication of steroid pulse therapy in this study persisted for more than 1 g/day for more than 3 months. In contrast to pediatric nephrotic syndrome, remission of proteinuria occurs far later than weeks in the majority of GN cases, as reflected by KDIGO recommending first-line treatment for 6 months for most types of GN [
3]. Likewise, the endpoints of clinical trials on IgAN are remission rates at 6 months after treatment, including the global study on the effectiveness of oral methylprednisolone in IgAN [
19] and two clinical trials comparing steroid plus ACEi with ACEi alone in proteinuria in IgAN [
20,
21]. Therefore, even when we had the pathologic diagnosis of GN, aggressive IS are indicated only when the disease is active despite treatment with less toxic management for months not weeks [
3]. If we consider nonsteroidal IS treatment, such as with cyclosporine or MMF, we need a clear indication, such as a pathologic diagnosis of IgAN with diffuse mesangial proliferation, as recommended by the Japanese guidelines, because these medications may accompany serious complications [
17].
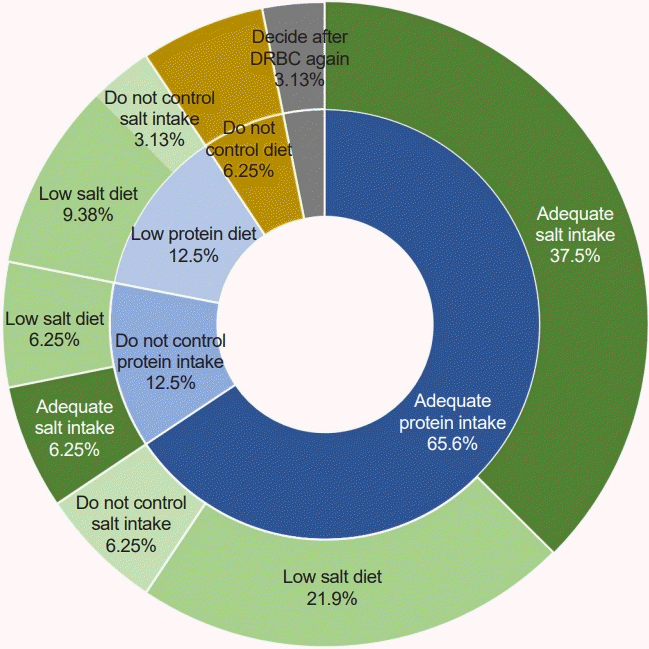
When managing children with AUA or kidney disease, one of the most common questions which patients ask to physicians is how to modify their diet. Often, the parents of children with supposedly kidney disease restrict salt and protein intake from the diet of the children, following the general recommendation of adult patients with CKD. However, there is no evidence supporting dietary restriction in children with AUA or GN if the patient does not have hypertension or edema [
4]. Salt restriction may be helpful in patients with hematuria due to hypercalciuria [
22]. In addition, as high salt intake is associated with high blood pressure [
23,
24], in children with CKD, salt restriction is recommended to the extent that it does not hinder growth in cases of hypertension [
25]. Otherwise, restricting salt intake does not help children with AUA. In contrast, restricting protein is not recommended even in children with CKD because there is no evidence that protein restriction can delay disease progression but only undermine growth [
26]. Therefore, Uauy et al. [
27] recommended that children consume as much protein as DRIs for their age. The most important consideration when recommending dietary modification for pediatric patients is that growth should not be hindered.
There were several limitations to this study. To the best of our knowledge, this is the first survey in Korea on clinical practice patterns for children with AUA. However, although most active members of the KSPN participated in the survey, the number of survey responders was too small, and not all members were included. In addition, the survey was conducted based on the clinical vignette, which may not have accurately reflected actual clinical practice in all situations. Therefore, future research should aim to survey a larger, more representative sample, and use more comprehensive survey instruments to obtain clearer results.
Although the number of survey responders was small, the survey provided valuable insight into the clinical approach of healthcare providers when managing children with AUA. Clinicians typically suspected IgAN when children showed hematuria and proteinuria, and were willing to perform a kidney biopsy. The most common treatment chosen was RAS inhibition, with or without steroids. Regarding protein intake, most responders recommended consuming DRIs. However, for salt intake, half of the responders recommended following a low-salt diet, while the other half recommended consuming as DRIs.
Children’s AUA is a common condition that has various causes, treatments, and prognoses. Since the treatment of AUA depends on the underlying etiology, such as GN, a definite diagnostic approach is needed, including a kidney biopsy. IS, which is usually included in the treatment of GN, have many adverse effects. Therefore, before using IS, it is necessary to confirm an accurate diagnosis and indications. Lastly, when recommending a diet to children with AUA or suspected GN, although evidence is insufficient, the priority should be to ensure that growth is not interrupted.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download