Abstract
Gastrointestinal (GI) bleeding is one of the most common conditions among patients visiting emergency departments in Korea. GI bleeding is divided into upper and lower GI bleeding, according to the bleeding site. GI bleeding is also divided into overt and occult GI bleeding based on bleeding characteristics. In addition, obscure GI bleeding refers to recurrent or persistent GI bleeding from a source that cannot be identified after esophagogastroduodenoscopy or colonoscopy. The small intestine is the largest part of the alimentary tract. It extends from the pylorus to the cecum. The small intestine is difficult to access owing to its long length. Moreover, it is not fixed to the abdominal cavity. When hemorrhage occurs in the small intestine, the source cannot be found in many cases because of the characteristics of the small intestine. In practice, small-intestinal bleeding accounts for most of the obscure GI bleeding. Therefore, in this review, we introduce and describe systemic approaches and examination methods, including video capsule endoscopy and balloon enteroscopy, that can be performed in patients with suspected small bowel bleeding in clinical practice.
Gastrointestinal (GI) bleeding is a very common condition in patients visiting outpatient clinics or emergency departments, leading to hospitalization. GI bleeding is continuously increasing due to the aging of the population and increasing drug use, including cardiovascular and neurologic drugs. According to a report by the Health Insurance Review and Assessment Service in Korea, the number of patients with GI bleeding increased by 30% over five years from 2011 to 2015. In the United States, GI bleeding contributes to over 513,000 admissions and 5 billion United States dollars in direct costs annually.1
GI bleeding is classified as upper and lower GI bleeding according to the bleeding site. Based on the ligament of Treitz located in the duodenojejunal flexure, upper GI bleeding is defined as hemorrhage that originates from the esophagus to the ligament of Treitz and lower GI bleeding as bleeding that originates from a site distal to the ligament of Treitz. Based on the characteristics of bleeding, GI bleeding is also classified as overt and occult. Overt GI bleeding shows visible blood loss with hematemesis, coffee-ground emesis, melena, or hematochezia. Occult GI bleeding shows no evidence of visible blood loss. It may manifest with symptoms of blood loss, anemia, positive fecal occult blood test results, or iron deficiency anemia. Obscure GI bleeding is defined as recurrent or persistent GI bleeding from a source that cannot be demonstrated after esophagogastroduodenoscopy (EGD) or colonoscopy, and can be either overt or occult.2,3
Obscure GI bleeding accounts for approximately 5% of all GI bleeding cases. One study using video capsule endoscopy (VCE) demonstrated that approximately 75% of patients diagnosed with obscure GI bleeding have bleeding from the small intestine.4 The small intestine is the longest section of the alimentary tract. Its length is approximately 5 to 8 m depending on the individual. Although the frequency of small-intestinal disease is less than that of stomach or colon diseases, if a disease occurs in the small intestine, it is difficult to approach for the following reasons: (1) it is deep in the abdominal cavity, (2) it is not fixed, (3) it has vigorous contractility, and (4) it has overlying loops. Practically, it is difficult for physicians to diagnose and treat patients with suspected small bowel diseases. Therefore, this review summarizes the systemic approaches for patients with presumed small bowel bleeding in clinical practice, especially those with presumed small bowel bleeding.
Understanding the causes of small bowel bleeding according to patient age or underlying disease is useful for diagnosing suspected small bowel bleeding. Regarding the causes of small bowel bleeding according to age, the most common causes of small bowel bleeding in patients over 40 years of age are vascular ectasias, tumors (e.g., GI stromal tumors, neuroendocrine tumors, adenocarcinomas, lymphomas, and metastases), and non-steroidal anti-inflammatory drugs (NSAIDs)-induced ulcers. The relatively common causes of small bowel bleeding in patients aged <40 years include inflammatory bowel diseases, polyposis syndromes, and Meckel’s diverticulum.
Although relatively infrequent, infections, ischemia, vasculitis, small intestine varices, intussusception, Dieulafoy’s lesion, aortoenteric fistula, and overlapping cyst are known to cause bleeding in the small intestine. Tumors (benign or malignant) may also occur in patients aged <40 years. Meckel's diverticulum is the most common cause of small-intestinal bleeding in children. Its prevalence decreases with age (Table 1).3,5-7
Most patients with obscure GI bleeding are hemodynamically stable, except for rare cases of aortic fistula, Dieulafoy’s lesion, and small-intestinal diverticulum. Therefore, careful history taking is important. Identifying the color and shape of the stool and the time of symptom onset can help determine which tests to be performed for diagnosis. Melena refers to bleeding from the GI tract above the ligament of Treitz. A Canadian study reported that in patients with obscure GI bleeding with melena, the cause of bleeding is twice as likely to be found in the proximal two-thirds of the small intestine.8 In addition, if enteroscopy is considered in patients with obscure GI bleeding with melena, information can be obtained to help decide whether to perform enteroscopy through the mouth or the anus.
Patients with long-term obscure GI bleeding, such as those with iron deficiency anemia without overt GI bleeding, are presumed to have bleeding due to a benign disease rather than malignancy. In this situation, diet, GI surgery including Roux-en-Y gastric bypass, menorrhagia, epistaxis, and blood loss due to hemodialysis can also be considered causes of small bowel bleeding. In addition, identifying comorbidities and medications in patients is useful for diagnosis. Congestive heart failure, chronic kidney disease, liver cirrhosis, aortic stenosis, and systemic sclerosis are known to cause vascular ectasia. Medications such as anticoagulants, antiplatelet agents, and NSAIDs are closely associated with bleeding. Therefore, physicians should identify the patients taking these medications.
Several examination methods are available for patients with suspected small bowel bleeding. Among these, repeated EGD and/or colonoscopy are easily accessible and important diagnostic methods for evaluating patients with suspected small bowel bleeding. Second-look EGD should be considered in cases of recurrent hematemesis, melena, or a previously incomplete examination. In addition, second-look colonoscopy should be considered in the setting of recurrent hematochezia and if a lower source is suspected.
Previous studies have reported that in patients who show no bleeding sources in conventional EGD and colonoscopy, bleeding sources could be found in 2% to 25% of patients on repeat EGD and 6% to 23% of patients on repeat colonoscopy.3,9-11 A recent retrospective study from Italy evaluated 290 patients with obscure GI bleeding and negative conventional endoscopic findings.12 The study was conducted using VCE. It was found that 30.3% (88 patients) had non-small bowel bleeding missed on EGD or colonoscopy.12 In other words, the bleeding source could be found in 30.3% of patients if a second EGD and/or colonoscopy was performed. If the performance status of patients is eligible for endoscopy, second-look endoscopy should be considered, as it could help diagnose obscure GI bleeding, including small bowel bleeding.
Computed tomography (CT) enterography is a combination of small bowel distension with a neutral or low-density oral contrast mixture and abdominopelvic CT during the enteric phase following the administration of an intravenous contrast.13 This technique provides specific visualization of the small bowel wall. It is notably valuable for the identification of masses and other structural lesions, including those in the small bowel.5 However, when intravenous contrast is administered, CT enterography is not a suitable test for patients with acute kidney injury or chronic kidney diseases.
A recent study investigated 1,087 patients with small bowel bleeding who underwent initial multiphasic CT enterography.14 The overall diagnostic yield was 31.6% (95% confidence interval [CI], 29.0%–35.0%). Diagnostic yields for patients with overt or occult positive fecal occult blood tests were 35.0% and 35.3%, respectively.14 In mass detection using multiphasic CT enterography, the sensitivity was 90.2%, with a positive predictive value of 98.2%.14 Multiphasic CT enterography showed the highest detection rate of small bowel masses such as neuroendocrine tumors, GI stromal tumors, and adenocarcinomas.
A meta-analysis investigated the clinical effectiveness of CT enterography in obscure GI bleeding. Eighteen studies (n=660) were included. The pooled diagnostic yield of CT enterography was 40% (95% CI, 33%–49%) in patients with obscure GI bleeding.15 When CT enterography and double-balloon enteroscopy (DBE) were compared, yields for CT enterography and DBE were 38% and 78%, respectively.15 When CT enterography and angiography were compared, yields for CT enterography and angiography were 64% and 60%, respectively.15 Finally, yields for CT enterography and VCE were 31% and 53%, respectively.15 When classified according to the types of lesions detected, diagnostic yields for vascular and inflammatory lesions were significantly different between CT enterography and VCE. However, those for neoplastic or other lesions were not significantly different.15 Therefore, CT enterography is useful for diagnosing small bowel bleeding in clinical practice. It helps diagnose small bowel bleeding; and can diagnose, differentiate, and rule out masses.
VCE is a non-invasive method that offers diagnostic imaging of the small intestine. VCE involves swallowing a disposable capsule with a data recorder by patients.16-18 A recent meta-analysis study on VCE (328 original articles with 86,930 enrolled patients) has reported that obscure GI bleeding (n=44,750) is the most common indication for VCE during the recent two decades, followed by clinical symptoms, Crohn’s disease, neoplastic lesions, and celiac disease.19 The detection rate of VCE was 59% for all indications, and 56% for obscure GI bleeding.19 In accordance with a recent meta-analysis, the diagnostic yields of VCE in patients with obscure GI bleeding were found to be 38% to 87%. Positive and negative predictive values of VCE for small bowel lesions were 94% to 97% and 83% to 100%, respectively.5 Depending on the findings of VCE, additional examinations (e.g., enteroscopy), surgery, and medical treatment were performed in 37% to 87% of patients.20,21 As a result, 50% to 66% of patients were considered to have no evidence of rebleeding during the follow-up period.20,21 Therefore, VCE is useful for the diagnosis and management of small bowel diseases, including small bowel bleeding.
A previous meta-analysis of 14 studies comparing VCE with other modalities in patients with obscure GI bleeding reported that the total diagnostic yields for VCE and push enteroscopy were 56% and 26% (p<0.001), respectively. For VCE and small bowel series, total diagnostic yields were 42% and 6% (p<0.001), respectively.22 Another meta-analysis compared VCE and DBE and revealed that pooled diagnostic yields for VCE and DBE were 62% and 56%, respectively (p=0.16).23 The odds ratio of CE was 1.39 (95% CI, 0.88–2.20; p=0.16) compared with DBE.23 Interestingly, the diagnostic yield for DBE conducted in patients with previously positive VCE was 75.0% (95% CI, 60.1%–90.0%), whereas in patients with previously negative VCE, the diagnostic yield was 27.5% (95% CI, 16.7%–37.8%).23 VCE showed a superior diagnostic yield to other modalities, indicating that VCE could be useful, particularly when additional examinations (e.g., enteroscopy including DBE) were performed. However, VCE is contraindicated in patients with dysphagia and intestinal obstruction. According to a meta-analysis, the pooled retention rate of VCE was 2.1% (95% CI, 1.5%–2.8%) in patients with suspected small bowel bleeding and 2.2% (95% CI, 0.9%–5.0%) for abdominal pain and/or diarrhea.24 Examination might be limited owing to visually affected capsule transit time, bowel preparation, intestinal peristalsis, air bubbles, and bile (Table 2).
Push enteroscopy can be performed using a colonoscope or a dedicated push enteroscope. It can be approached from the distal duodenum and proximal jejunum approximately 50 to 150 cm beyond the ligament of Treitz by peroral insertion.25 The diagnostic yield of push enteroscopy was approximately 41% to 78% in previous studies. The most commonly detected lesions were vascular ectasias.26 Push enteroscopy can examine without additional equipment, unlike device-assisted enteroscopy. Therefore, it can be attempted in patients with suspected proximal small bowel bleeding. In other words, push enteroscopy is difficult to perform, except in the proximal part of the small bowel. Therefore, when mid or distal small bowel bleeding is suspected, DBE is more helpful than push enteroscopy.
Diagnostic yields of DBE were 60% to 80% in patients with presumed small bowel bleeding and other small bowel diseases.3 The successful control rate of endoscopic therapeutic procedures ranges from 40% to 73% in those patients.3,27,28 In a meta-analysis including 17 studies, the sensitivity, specificity, positive predictive value, and negative predictive value of DBE were 0.84 (95% CI, 0.82–0.86), 0.92 (95% CI, 0.89–0.94), 11.29 (95% CI, 4.83–26.40), and 0.20 (95% CI, 0.15–0.27), respectively.29 DBE is a more invasive examination than VCE. However, the advantage of DBE is that the disease can be treated at the same time as the examination. Although the difference was not statistically significant, the diagnostic yield of DBE (42.9%) was lower than that of VCE (59.4%) in a pilot study.30 However, biopsy or endoscopic treatment was possible for most small bowel lesions in the DBE group. Nonetheless, DBE can cause complications, such as perforation, bleeding, pancreatitis, aspiration pneumonia, and even death. Therefore, patient safety should be prioritized during the examination (Table 2). Figure 1 shows the management algorithm used for patients with suspected small bowel bleeding, as suggested by the authors.
Only a decade or two ago, accurate diagnosis and management of patients with obscure GI bleeding or suspected small bowel bleeding was difficult. However, advances in endoscopy and imaging technologies have increased the diagnostic yield for these patients. The development of DBE has enabled the endoscopic treatment of patients with small bowel bleeding. Understanding patients through detailed history-taking is a basic skill for physicians. When conducting examinations, physicians need to know the conditions of patients and indications, contraindications, advantages, and disadvantages of each examination before proceeding with the diagnosis and treatment. Nevertheless, in clinical practice, some patients with suspected small bowel bleeding have negative results in all these examinations. In this case, a close follow-up with sufficient mucosal protective agents may be performed in patients without overt GI bleeding. Science is advancing at a stunning speed. Artificial intelligence and machine learning are being researched by combining them with medical technologies. In the future, small bowel bleeding is expected to become a solvable disease rather than an unsolvable one.
REFERENCES
1. Saltzman JR. Burden and costs of gastrointestinal disease in the U.S. [Internet]. US: NEJM Journal Watch;2018. [cited 2022 Aug 26]. Available from: https://www.jwatch.org/na47723/2018/10/23/burden-and-costs-gastrointestinal-disease-us/.
2. Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007; 133:1697–1717.
3. Gerson LB, Fidler JL, Cave DR, et al. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015; 110:1265–1287.
4. Pasha SF, Leighton JA, Das A, et al. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008; 6:671–676.
5. Havlichek DH, Kamboj AK, Leggett CL. A practical guide to the evaluation of small bowel bleeding. Mayo Clin Proc. 2022; 97:146–153.
6. Lee YK, Oh TK, Choi A, et al. A case of Henoch Schoenlein purpura with gastrointestinal bleeding due to jejunal ulcer by capsule endoscopy. Kosin Med J. 2012; 27:45–49.
7. Yoo AY, Lee BJ, Kim WS, et al. Clinicopathological features of small bowel tumors diagnosed by video capsule endoscopy and balloon-assisted enteroscopy: a single center experience. Clin Endosc. 2021; 54:85–91.
8. Zhu CN, Friedland J, Yan B, et al. Presence of melena in obscure gastrointestinal bleeding predicts bleeding in the proximal small intestine. Dig Dis Sci. 2018; 63:1280–1285.
9. Descamps C, Schmit A, Van Gossum A. "Missed" upper gastrointestinal tract lesions may explain "occult" bleeding. Endoscopy. 1999; 31:452–455.
10. Fry LC, Bellutti M, Neumann H, et al. Incidence of bleeding lesions within reach of conventional upper and lower endoscopes in patients undergoing double-balloon enteroscopy for obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2009; 29:342–349.
11. Lara LF, Bloomfeld RS, Pineau BC. The rate of lesions found within reach of esophagogastroduodenoscopy during push enteroscopy depends on the type of obscure gastrointestinal bleeding. Endoscopy. 2005; 37:745–750.
12. Innocenti T, Dragoni G, Roselli J, et al. Non-small-bowel lesions identification by capsule endoscopy: a single centre retrospective study. Clin Res Hepatol Gastroenterol. 2021; 45:101409.
13. Ilangovan R, Burling D, George A, et al. CT enterography: review of technique and practical tips. Br J Radiol. 2012; 85:876–886.
14. Deepak P, Pundi KN, Bruining DH, et al. Multiphase computed tomographic enterography: diagnostic yield and efficacy in patients with suspected small bowel bleeding. Mayo Clin Proc Innov Qual Outcomes. 2019; 3:438–447.
15. Wang Z, Chen JQ, Liu JL, et al. CT enterography in obscure gastrointestinal bleeding: a systematic review and meta-analysis. J Med Imaging Radiat Oncol. 2013; 57:263–273.
16. ASGE Technology Committee, Wang A, Banerjee S, et al. Wireless capsule endoscopy. Gastrointest Endosc. 2013; 78:805–815.
17. Abutalib H, Yano T, Shinozaki S, et al. Roles of capsule endoscopy and balloon-assisted enteroscopy in the optimal management of small bowel bleeding. Clin Endosc. 2020; 53:402–409.
18. Elli L, Centorrino E, Costantino A, et al. Capsule enteroscopy versus small-bowel ultrasonography for the detection and differential diagnosis of intestinal diseases. Clin Endosc. 2022; 55:532–539.
19. Cortegoso Valdivia P, Skonieczna-Żydecka K, Elosua A, et al. Indications, detection, completion and retention rates of capsule endoscopy in two decades of use: a systematic review and meta-analysis. Diagnostics (Basel). 2022; 12:1105.
20. Lai LH, Wong GL, Chow DK, et al. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006; 101:1224–1228.
21. Estévez E, González-Conde B, Vázquez-Iglesias JL, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006; 18:881–888.
22. Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005; 100:2407–2418.
23. Teshima CW, Kuipers EJ, van Zanten SV, et al. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011; 26:796–801.
24. Rezapour M, Amadi C, Gerson LB. Retention associated with video capsule endoscopy: systematic review and meta-analysis. Gastrointest Endosc. 2017; 85:1157–1168.
25. ASGE Technology Committee, DiSario JA, Petersen BT, et al. Enteroscopes. Gastrointest Endosc. 2007; 66:872–880.
26. Linder J, Cheruvattath R, Truss C, et al. Diagnostic yield and clinical implications of push enteroscopy: results from a nonspecialized center. J Clin Gastroenterol. 2002; 35:383–386.
27. Yamamoto H, Kita H, Sunada K, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004; 2:1010–1016.
28. May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005; 62:62–70.
29. Brito HP, Ribeiro IB, de Moura DT, et al. Video capsule endoscopy vs double-balloon enteroscopy in the diagnosis of small bowel bleeding: a systematic review and meta-analysis. World J Gastrointest Endosc. 2018; 10:400–421.
30. Xin L, Liao Z, Jiang YP, et al. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011; 74:563–570.
Fig. 1.
Management algorithm for suspected small bowel bleeding. EGD, esophagogastroduodenoscopy; CT, computed tomography; VCE, video capsule endoscopy; DAE, device-assisted enteroscopy.
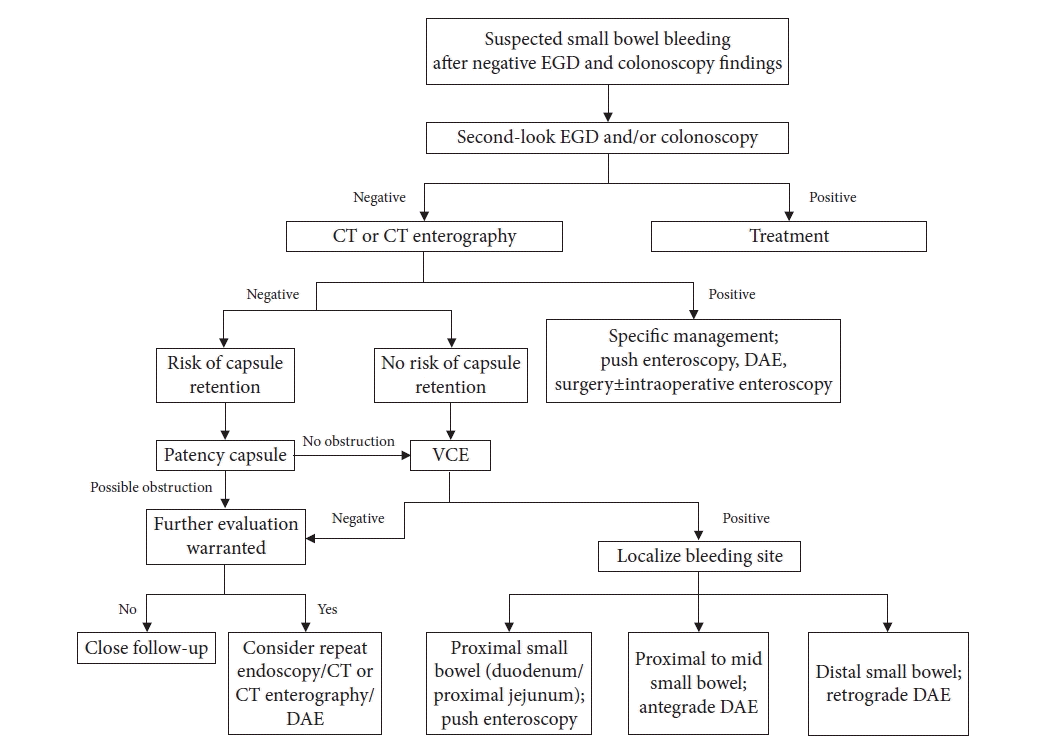
Table 1.
List of causes of small bowel bleeding
Table 2.
Indications and contraindications of video capsule endoscopy and enteroscopy




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download