Abstract
Background/Aims
We compared outcomes between use of 15 vs. 20 mm lumen-apposing metal stents (LAMSs) in endoscopic ultrasound-guided gastroenterostomy (EUS-GE) for gastric outlet obstruction.
Methods
Databases were queried for studies that used LAMS for EUS-GE to relieve gastric outlet obstruction, and a proportional meta-analysis was performed.
Results
Thirteen studies were included. The 15 mm and 20 mm LAMS had pooled technical success rates of 93.2% (95% confidence interval [CI], 90.5%–95.2%) and 92.1% (95% CI, 68.4%–98.4%), clinical success rates of 88.6% (95% CI, 85.4%–91.1%) and 89.6% (95% CI, 79.0%–95.1%), adverse event rates of 11.4% (95% CI, 8.1%–15.9%) and 14.7% (95% CI, 4.4%–39.1%), and reintervention rates of 10.3% (95% CI, 6.7%–15.4%) and 3.5% (95% CI, 1.6%–7.6%), respectively. Subgroup analysis revealed no significant differences in technical success, clinical success, or adverse event rates. An increased need for reintervention was noted in the 15 mm stent group (pooled odds ratio, 3.59; 95% CI, 1.40–9.18; p=0.008).
Gastric outlet obstruction (GOO) is a clinical condition that presents with symptoms of luminal obstruction such as nausea, vomiting, epigastric pain, and the inability to tolerate oral nutrition.1 The level of obstruction can be present in the distal stomach, pylorus, or small bowel. The benign causes of GOO include peptic ulcer disease, hypertrophic pyloric obstruction, caustic ingestion, and iatrogenic etiologies. In contrast, malignant causes of GOO include pancreatic, gastric, gallbladder, duodenal or ampullary cancers.2 Management of the symptoms of GOO include making the patient avoid oral intake, administering intravenous fluids to correct volume depletion and correct electrolyte abnormalities, a parenteral proton pump inhibitor to decrease gastric secretions, medications for pain and nausea as needed, and a nasogastric tube for gastric decompression and symptom relief. However, definitive management of GOO is only achieved when the mechanical obstruction is resolved.
Relieving mechanical obstruction in GOO traditionally involves surgical gastrojejunostomy (S-GE) or enteral stenting (ES). S-GE has a complication rate of 13% to 55% and a mortality rate of 2% to 36%.3 Further limiting the utility of S-GE is the poor clinical status, including malnutrition among GOO patients, that predisposes to poor outcomes, protracted recovery delaying chemotherapy initiation in malignant obstructions, gastroparesis, and procedure-associated costs.4-6 ES can be offered as an alternative to S-GE and is associated with a quicker oral tolerance, decreased morbidity, lower incidence of gastroparesis, and shorter length of stay compared to S-GE.7 Yet, the clinical course of ES can be complicated due to need for recurrent interventions due to stent migration or obstruction due to tumor ingrowth or tissue hyperplasia.8-10 As such, ES is better suited for patients with shorter life expectancy in whom reintervention is less likely.11,12 endoscopic ultrasound (EUS)-guided gastroenterostomy (EUS-GE) has emerged as an alternative therapeutic option with more durable patency.
In EUS-GE, the obstruction is bypassed using EUS to identify a point in the small bowel past the obstruction and create a bypass from the stomach with a lumen-apposing metal stent (LAMS).13 Since a covered stent is positioned between the stomach and small bowel away from the site of the tumor, reintervention is rarely required because of lack of risk of stent occlusion from the tumor or tissue hyperplasia. Pooled technical, clinical, and adverse events and the need for reintervention rates for this procedure are 90% to 93%, 90%, 5% to 12%, and 9% to 11%, respectively.5,13 Therefore, EUS-GE may be an option for the treatment of GOO. However, the optimal technique and tools used during the procedure are yet to be determined.
LAMS is currently considered the first-line treatment for EUS-GE. Historically, the 15 mm LAMS has been utilized for EUS-GE, but the 20 mm LAMS has recently become available as an alternative stent option. This offers the theoretical advantage of an increase in luminal area compared to the 15 mm LAMS, which may offer a benefit in outcomes such as long-term lumen patency and fewer dietary restrictions.14 Conversely, the 20 mm LAMS may be more difficult to deploy in a small bowel loop due to a larger flange diameter, which may increase the risk of adverse events. This study aimed to perform a meta-analysis to evaluate the efficacy and safety of 20 mm LAMS and compare it with 15 mm LAMS for the treatment of GOO.
PubMed, Embase, and Google Scholar databases were queried through November 2021 to identify studies that used 15 or 20-mm LAMS for EUS-GE to relieve GOO. The systematic literature review was independently performed by two authors (SV and RS) using the following search terms: “endoscopic ultrasound and gastric outlet obstruction,” “endoscopic ultrasound and pyloric obstruction,” “endoscopic ultrasound-guided gastroenterostomy (EUS-GE),” “endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ),” and “endoscopic ultrasound-guided gastroduodenostomy.” endoscopic ultrasound-guided gastroduodenostomy. Abstracts were reviewed for appropriateness (SV and RS), and any differences were resolved by other authors (SK, SB, and SA).
Eligible studies were randomized controlled trials, cross-sectional studies, or cohort studies that used 15 or 20-mm LAMS for EUS-GE to relieve GOO. All relevant articles were included, regardless of the year of publication, publication status, or language, although individual case reports were not included. Multiple EUS-GE procedures, including assisted and unassisted techniques, have been described in literature. Differences in technique were not an exclusionary criterion. Studies were excluded if they did not investigate this methodology in adults, did not report on which stent size was used, or had insufficient data. In cases of suspicion of duplicated patients (i.e., from publications from shared authors), in order to preserve independence of observations, data from the most recent and/or most appropriate comprehensive report were retained.
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist was followed.15 The Newcastle-Ottawa score (NOS) was used to evaluate the quality of the cohort studies based on the selection of study groups, comparability of the groups, and the analysis of outcome concerning exposure of interest (maximum score of 9, with ≥5 indicating high quality).16 Two authors (SV and RS) independently assessed the risk of bias and study quality. A funnel plot of the effect size against the sample size was produced for the included studies to evaluate the presence of publication bias.
A structured data collection form was created to extract data from each study. Data collected included the title of the study, publication year, country of origin, number of participants, patient demographics, and primary and secondary outcomes. These outcomes included the primary outcome of technical success (successful EUS-directed deployment of the LAMS) and secondary outcomes, including clinical success (ability to tolerate oral intake after the procedure as defined by individual authors), adverse events reported during follow-up, and reinterventions required during follow-up. Although the American Society of Gastrointestinal Endoscopy has recommendations for the classification of adverse events, these definitions were not uniformly followed by the included studies.17 Similarly, a uniform definition of reintervention was lacking. Therefore, the reported adverse events and reinterventions were logged and grouped together. When articles did not describe any reinterventions but did describe adverse events, we attempted to contact the corresponding authors to confirm that no reinterventions were required in their study population. When contact could not be made with the corresponding authors, it was assumed that no reinterventions were required in their study population for the purposes of this meta-analysis. When heterogeneity was found, subgroup analysis was performed if significant power was available. Both benign and malignant cases were included in the same analysis. This was performed for several reasons, including many papers that did not differentiate their results based on etiology (benign vs. malignant), which would preclude subgroup analysis based on etiology. Only a small number of cases would have been excluded if benign GOO were an exclusion criterion. Second, the studies did not describe differential techniques based on the etiology of GOO. Since etiology did not appear to affect technique in the studies, it was decided that etiology should not be a factor in subgroup analysis. Two authors (SV and RS) independently extracted the data for each article. This data extraction was confirmed by other authors (SB, SK, and SA), and any disagreements were resolved.
Statistical analysis was performed using the Comprehensive Meta-Analysis tool ver. 2.2.057 (BioStat, Englewood, NJ, USA). This meta-analysis calculated pooled proportions with 95% confidence intervals (CIs) for each of the primary and secondary outcomes using a random-effects model for each stent size. Subgroup analysis was used to compare pooled rates for each outcome using Mantel–Haenszel pooled odds ratios (ORs) in a random-effects model. Statistical heterogeneity was assessed using the I2 statistic and publication bias was assessed using funnel plots. A p-value <0.05 was considered as statistically significant.
In the initial search, 826 records were retrieved using the search strategy shown in the PRISMA flowchart in Figure 1. After applying the exclusion criteria, 26 articles were reviewed. After this, 13 studies remained for a total of 685 patients.10,14,18-28 Of these, 499 patients received the 15 mm LAMS while 186 patients received the 20 mm LAMS.
Baseline characteristics and quality assessments are reported in Table 1.10,14,18-28 Of the included studies, two were prospective studies and 11 were retrospective, of which six were multicenter studies. All studies were of good quality according to the NOS. Visual inspection of the funnel plot diagrams did not reveal any obvious publication bias for the primary and secondary outcomes (Supplementary Fig. 1). Heterogeneity was found to be low to moderate in the 15 mm LAMS group overall, with I2 values for technical, clinical, and adverse events, and reintervention rates of 0%, 0%, 31.5%, and 47.4%, respectively. On the other hand, an increased heterogeneity was found in the 20 mm LAMS group overall with I2 values for technical, clinical, and adverse events, and reintervention rates of 84.4%, 45.4%, 85.7%, and 0%, respectively.
Table 1 also reports the individual number of cases that had technical and clinical success and how many adverse events and reinterventions were required in each study, which is also represented through Forrest plots in Figure 2. Table 2 presents the pooled event rate for each outcome. Supplementary Table 1 further describes the occurrence of each of the described adverse events and reintervention events that occurred throughout each of the included studies.
Technical success was achieved in 469 of 499 patients who received the 15 mm LAMS with a pooled technical success rate of 93.2% (95% CI, 90.5%–95.2%). In the 20 mm LAMS group, this was achieved in 172 of 186 patients, with a pooled technical success rate of 92.1% (95% CI, 68.4%–98.4%). No difference was observed in the odds of technical success between the two stent sizes (pooled OR, 1.27; 95% CI, 0.66–2.46; p=0.47).
Clinical success was achieved in 444 of 499 patients who received the 15 mm LAMS with a pooled clinical success rate of 88.6% (95% CI, 95.4%–91.1%). In the 20 mm LAMS group, this was achieved in 163 of 186 patients, with a pooled clinical success rate of 89.6% (95% CI, 79.0%–95.1%). Again, no difference was observed in the odds of clinical success between the two stent size options (pooled OR, 1.14; 95% CI, 0.68–1.91; p=0.62).
Adverse events occurred in 51 of 499 patients who received the 15 mm LAMS with a pooled adverse event rate of 11.4% (95% CI, 8.1%–15.9%). These occurred in 28 of 186 patients who received the 20 mm LAMS with a pooled adverse event rate of 14.7% (95% CI, 4.4%–39.1%). No difference was observed in the odds of adverse events between the two stent size options; however, a trend toward increased adverse events was noticed in the 20 mm LAMS group (pooled OR, 0.64; 95% CI, 0.39–1.05; p=0.08).
Reinterventions occurred in 45 of 499 patients who received the 15 mm LAMS with a pooled reintervention rate of 10.3% (95% CI, 6.7%–15.4%). Conversely, reinterventions occurred in 5 of 186 patients, with a pooled reintervention rate of 3.5% (95% CI, 1.6%–7.6%). Statistically significant increased odds were observed for reintervention among those who received the 15 mm stent compared to those who received the 20 mm stent (pooled OR, 3.59; 95% CI, 1.40–9.18; p=0.008).
While previous meta-analyses demonstrated the safety and efficacy of EUS-GE for GOO, our meta-analysis provides novel information by comparing the outcomes with the use of 15 mm versus 20 mm LAMS.5,13 We demonstrated that both the 15 mm and 20 mm stents appear to be safe and efficacious because no difference was observed in technical success, clinical success, or adverse event rates. However, our results suggest that the rate of reintervention may be lower when a 20 mm stent is used.
Two recent systematic reviews and meta-analyses have described the results of using EUS-GE for GOO.5,13 McCarty et al.5 report pooled technical success in 93% (95% CI, 88%–96%), clinical success in 90% (95% CI, 85%–93%), adverse events in 6% (95% CI, 3%–11%), and a reintervention rate of 11% (95% CI, 7%–17%). Iqbal et al.13 described pooled technical success in 92% (95% CI, 88%–95%), clinical success in 90% (95% CI, 85%–94%), adverse events in 12% (95% CI, 8%–16%), and a reintervention rate of 9% (95% CI, 6%–13%). Our findings for both stent sizes were consistent with the findings of these studies. Notably, the 20 mm LAMS subgroup had a significantly lower reintervention rate of 3.5%.
Clinical success rates between the two stent sizes were similar, with 88.6% (95% CI, 0.85%–0.91%) in the 15 mm LAMS group and 89.6% (95% CI, 0.79–0.95) in the 20 mm LAMS group (p=0.62). In a study that directly compared 15 mm vs. 20 mm LAMS, Bejjani et al.27 found that while technical success was similar between the groups, a higher proportion of the patients in the 20 mm LAMS group tolerated soft/complete diet compared to those in the 15 mm LAMS group (91.2% vs. 81.2%, p=0.04). Sobani et al.14 similarly remarked that a significant proportion of the previous studies using the 15 mm LAMS relayed clinical success in patients limited to liquid or soft diets. In their study, Sobani et al.14 defined clinical success with their 20 mm LAMS as tolerating a regular diet, attained in 93.5% of their participants. It is theorized that the wider lumen in a 20 mm LAMS better permits a regular diet for multiple reasons: (1) it is closer to physiologic gastric outlet size of 20 to 23 mm, (2) it mirrors the size of stents used in ES, and the anastomosis made in S-GE, and (3) it has a lower likelihood of impaction from food or tissue.14,29
As the 20 mm LAMS is larger, it could theoretically be expected to be more difficult to deploy and increase the risk of adverse events. For example, the outer flange of the 20 mm stent was 29 mm, as opposed to 24 mm in the 15 mm stent, which increased the margin of error of deployment into the jejunal loop. The adverse event rate for the 15 mm LAMS group was 11.4% (95% CI, 0.08–0.16) compared to 14.7% (95% CI, 0.04%–0.39%) in the 20 mm LAMS group. Although this difference was a notable trend, it did not reach statistical significance (p=0.08). While the relative numbers of each of these adverse events were not high enough to meet significance, adverse events in the 20 mm group were higher and included certain complications not seen in the 15 mm group such as infection/peritonitis, postprocedural bleeding, metal stent cutting of the guidewire, stent migration, a jejunal ulcer three months after the procedure, and two other not-described events. Theoretically, this adverse event rate may decrease with future studies as advanced endoscopists gain more experience with the procedure and the new stent size, as has been demonstrated in other procedures utilizing LAMS.30-32
A significant difference was observed in the reintervention rates between the two groups as the 15 mm LAMS group was 10.3% (95% CI, 0.07–0.15) and the 20 mm LAMS group was 3.5% (95% CI, 0.016–0.076). Subgroup analysis revealed a significant difference (p=0.008). All reinterventions in the 20 mm LAMS group were performed due to GOO recurrence or stent obstruction. In contrast, the 15 mm LAMS group had additional reinterventions including the need for percutaneous tube placement, tissue overgrowth, delayed gastric emptying, laparotomy for leakage at the LAMS site, and other events. This significant difference in reintervention rate contrasts with the findings in the Bejjani et al.27 study, which found no significant difference between the two stent sizes within their own study. We believe the findings in our study are related to the addition of two studies that did not have any reinterventions in their study populations.14,28 Additional heterogeneity may be hidden in the methodology of the studies as well, as the Bejjani et al.27 study, which had all the reinterventions in the 20 mm LAMS subgroup, was a multicenter study with multiple performing endoscopists, whereas the Sobani et al.14 and Xu et al.28 studies had 20 mm LAMS placed by a single or two endoscopists, respectively. Operator differences may have led to the stent being placed in different locations in the stomach or jejunum. This may have affected the ability to tolerate the diet and subsequent occlusion and reintervention rates. In addition, operator error or experience may have confounded the results. Differences in the study populations may also be confounding, as the first study was conducted across multiple centers, whereas the latter two were limited to their study sites.
This study has several limitations. First, much of the data were retrospectively collected. Additionally, differences in patient populations may lead to bias that is not captured through funnel plot assessment of publication bias. Increased heterogeneity was noted in the analysis of the 20 mm LAMS for technical success and adverse event rates, suggesting differences in study populations, measurement of outcomes, or analytical methods. On the other hand, there was low-moderate and no heterogeneity noted in the analysis of the clinical success and reintervention rates in the analysis of the 20 mm LAMS, suggesting that these possible differences may not be present or were not similarly affecting the measurement of these outcomes. For example, multiple EUS-GE techniques were used in the study. Notably, the techniques used in the 20 mm LAMS group included direct EUS-GE, balloon-assisted, and double balloon-assisted, although exact numbers for each subgroup were not reported, so subgroup analysis by technique within 20 mm LAMS could not be performed (amongst other possible variables for meta-regression). Future analyses should attempt to clarify which EUS-GE technique yields the best results. As a novel procedure with a notable learning curve, there may be temporal trends in adverse events and reintervention rates. As can be seen by the timeline of studies, a future analysis may observe an “era” effect as the proceduralists gain more experience with the techniques and patient selection. However, because of the small sample size, we were unable to evaluate these factors. Additionally, there may be increased heterogeneity due to the benign or malignant etiology of GOO. It was decided not to perform subgroup analysis based on benign or malignant causes of GOO, as many manuscripts did not differentiate their results based on etiology, which would preclude subgroup analysis, whereas others did not include any benign cases. Indeed, only one study27 differentiated the results based on etiology. As many studies did not differentiate the technique or stent size based on etiology, it was felt that this was not likely a driving factor in the choice of stent size, although this may be further investigated in the future. Other possibilities for heterogeneity include differences between LAMS types (though authors described using “Hot Axios stents” in their methods, so it seems likely that the majority of stents seem to have been Boston Scientific LAMS [Axios]) and gastric or duodenal location of obstruction (most studies described using EUS-GE for GOO without describing duodenal locations). Second, while clinical success rates were reported in all included studies, they were not objectively defined in many cases. This obfuscates the validity of the reported pooled clinical success rates reported above, and future studies should consider using a validated, objective scoring system (such as the GOO scoring system [GOOSS]).33 Future studies should aim to adhere by the GOOSS to standardize the definition of clinical success, such as requiring a score of 2 after a certain number of days post-procedure. The GOOSS score was only reported in three studies in the 15 mm arm19,24,27 and two in the 20 mm arm.27,28 As such, pooled analysis was omitted because it would not provide clinically meaningful evidence. Third, many studies have included similar authors. Therefore, we cannot definitively exclude potentially duplicate patients, and attempts were made to do so. This may have introduced bias into the results of this study. In addition, it may be reasonable to assume that the choice of stent size is most likely to be influenced by clinical anatomy; this study provides reassurance that anatomy at the time of deployment should be more important than differences in outcomes, as there may only be a marginal benefit in reintervention rate with a 20 mm LAMS. Fourth, an interesting variable that may influence stent choice is the patency duration between the LAMSs. Unfortunately, this has not been described uniformly in previous studies. Some described time frames within the hospital stay, while others extended their follow-up period to up to a year. Fifth, the studies by Bejjani et al.27 and Sobani et al.14 reported that some of their authors are consultants for the company Boston Scientific, which may lead to a bias towards using their stents, but the company manufactures both stent sizes, so it is unlikely that this source of funding affected stent size choice. Finally, the included studies were of modest size; therefore, larger studies may be required to elucidate the theoretical benefits of either stent size option. Although a statistically significant difference was found in the reintervention rate, the results of the meta-analysis are based on a small number of studies and a pool of patients, which may have influenced the final results.
Despite these limitations, our study has several strengths. The cumulative sample size was large, and all the included studies were of good quality. There appeared to be low-to-moderate heterogeneity between most studies and no publication bias. No prior analyses have reported on the outcomes between the different stent size options or grouped adverse outcomes and reinterventions by each stent size option. The findings reported in this meta-analysis provide a step forward in understanding and optimizing EUS-GE for GOO.
In conclusion, our meta-analysis suggests lack of difference in technical success, clinical success, or adverse event rates between 15 mm and 20 mm LAMS use. However, there may be less reintervention required when a larger stent is used. Given all the variables involved, it is difficult to use one stent size over another, based on the varied nature of the studies describing a relatively new procedure and technique. Currently, it is more likely to be a challenge with the anatomy and learning curve that drives the decision to choose one stent size over another. Future studies should aim to prospectively compare S-GE, ES, and EUS-GE, while considering the information obtained here regarding the influence of stent size on outcomes. Although EUS-GE is a technically challenging procedure, it can be an effective and safe option for GOO, and the choice of stent diameter should be individualized.
Supplementary Material
Supplementary Fig. 1. Funnel plots demonstrating no obvious publication bias for technical success, clinical success, adverse events, and reintervention events between 15 mm and 20 mm lumen-apposing metal stents.
Supplementary Table 1. The types of adverse and reintervention events are summarized in all of the included studies
Supplementary materials related to this article can be found online at https://doi.org/10.5946/ce.2022.299.
Notes
Conflicts of Interest
Sunil Amin was a consultant for Boston Scientific. The other authors have no potential conflicts of interest.
Acknowledgments
Advice on data analysis was provided by the in-house statistician, Thilani Samarakoon, whose aid was greatly appreciated.
Author Contributions
Conceptualization: SV, SB, SK, SA; Data curation: SV, RS; Formal analysis: SV, RS, SB, SK, SA; Investigation: SV, RS; Methodology: SV, RS; Resources: SK; Software: SV, RS, SK; Supervision: SB, SK, SA; Validation: SB, SK, SA; Writing–original draft: SV, RS; Writing–review & editing: all authors.
REFERENCES
1. Koop AH, Palmer WC, Stancampiano FF. Gastric outlet obstruction: a red flag, potentially manageable. Cleve Clin J Med. 2019; 86:345–353.
2. Stefanovic S, Draganov PV, Yang D. Endoscopic ultrasound guided gastrojejunostomy for gastric outlet obstruction. World J Gastrointest Surg. 2021; 13:620–632.
3. Potz BA, Miner TJ. Surgical palliation of gastric outlet obstruction in advanced malignancy. World J Gastrointest Surg. 2016; 8:545–555.
4. No JH, Kim SW, Lim CH, et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc. 2013; 78:55–62.
5. McCarty TR, Garg R, Thompson CC, et al. Efficacy and safety of EUS-guided gastroenterostomy for benign and malignant gastric outlet obstruction: a systematic review and meta-analysis. Endosc Int Open. 2019; 7:E1474–E1482.
6. Itoi T, Baron TH, Khashab MA, et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc. 2017; 29:495–502.
7. Hosono S, Ohtani H, Arimoto Y, et al. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007; 42:283–290.
8. Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010; 71:490–499.
9. Jeurnink SM, Steyerberg EW, Hof Gv, et al. Gastrojejunostomy versus stent placement in patients with malignant gastric outlet obstruction: a comparison in 95 patients. J Surg Oncol. 2007; 96:389–396.
10. Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019; 33:3404–3411.
11. Adler DG. Should patients with malignant gastric outlet obstruction receive stents or surgery? Clin Gastroenterol Hepatol. 2019; 17:1242–1244.
12. Troncone E, Fugazza A, Cappello A, et al. Malignant gastric outlet obstruction: which is the best therapeutic option? World J Gastroenterol. 2020; 26:1847–1860.
13. Iqbal U, Khara HS, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: a systematic review and meta-analysis. Endosc Ultrasound. 2020; 9:16–23.
14. Sobani ZA, Paleti S, Rustagi T. Endoscopic ultrasound-guided gastroenterostomy using large-diameter (20 mm) lumen apposing metal stent (LLAMS). Endosc Int Open. 2021; 9:E895–E900.
15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.
16. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute;2019. [cited 2021 Jul 23]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
17. ASGE Standards of Practice Committee, Early DS, Acosta RD, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013; 77:839–843.
18. Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016; 4:E276–E281.
19. Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016; 65:193–195.
20. Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017; 5:E275–E281.
21. Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an international collaborative study. J Clin Gastroenterol. 2017; 51:896–899.
22. Kerdsirichairat T, Yang J, Gutierrez OI, et al. Su1362 Long-term outcomes of endoscopic ultrasound-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction: A 4-year cohort. Gastrointest Endosc. 2018; 87(6S):AB320–AB321.
23. Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc. 2018; 87:1215–1221.
24. Kerdsirichairat T, Irani S, Yang J, et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019; 7:E144–E150.
25. Bondi G, Bazarbashi AN, Abbas AM, et al. Endoscopic gastroenterostomy versus surgical gastrojejunostomy for the treatment of gastric outlet obstruction in patients with peritoneal carcinomatosis: a retrospective comparative study. Gastrointest Endosc. 2020; 91(6S):AB303.
26. Nguyen NQ, Hamerski CM, Nett A, et al. Endoscopic ultrasound-guided gastroenterostomy using an oroenteric catheter-assisted technique: a retrospective analysis. Endoscopy. 2021; 53:1246–1249.
27. Bejjani M, Ghandour B, Subtil JC, et al. Clinical and technical outcomes of patients undergoing endoscopic ultrasound-guided gastroenterostomy using 20-mm vs. 15-mm lumen-apposing metal stents. Endoscopy. 2022; 54:680–687.
28. Xu G, Shen Y, Lv Y, et al. Safety and efficacy of endoscopic ultrasound-guided gastroenterostomy using double balloon occlusion methods: a clinical retrospective study in 36 patients with malignant gastric outlet obstruction. Endosc Int Open. 2020; 8:E1690–E1697.
29. Madanat L, Saumoy M, Sharaiha RZ. Endoscopic gastrojejunostomy: bigger is better. Endoscopy. 2018; 50:E331–E332.
30. Tyberg A, Kedia P, Tawadros A, et al. EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE): the first learning curve. J Clin Gastroenterol. 2020; 54:569–572.
31. Tyberg A, Kats D, Choi A, et al. Endoscopic ultrasound guided gastroenterostomy: what is the learning curve? J Clin Gastroenterol. 2021; 55:691–693.
32. Tyberg A, Jha K, Shah S, et al. EUS-guided gallbladder drainage: a learning curve modified by technical progress. Endosc Int Open. 2020; 8:E92–E96.
33. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002; 97:72–78.
Fig. 1.
Literature review flowchart. Describes a methodology for literature search and exclusion criteria for inclusion into meta-analysis. LAMS, lumen-apposing metal stent.
Fig. 2.
Forest plots demonstrating technical success, clinical success, adverse events, and reintervention events between 15 mm and 20 mm lumen-apposing metal stents. Included tables report on group event rate, 95% confidence intervals (CIs), and p-values.
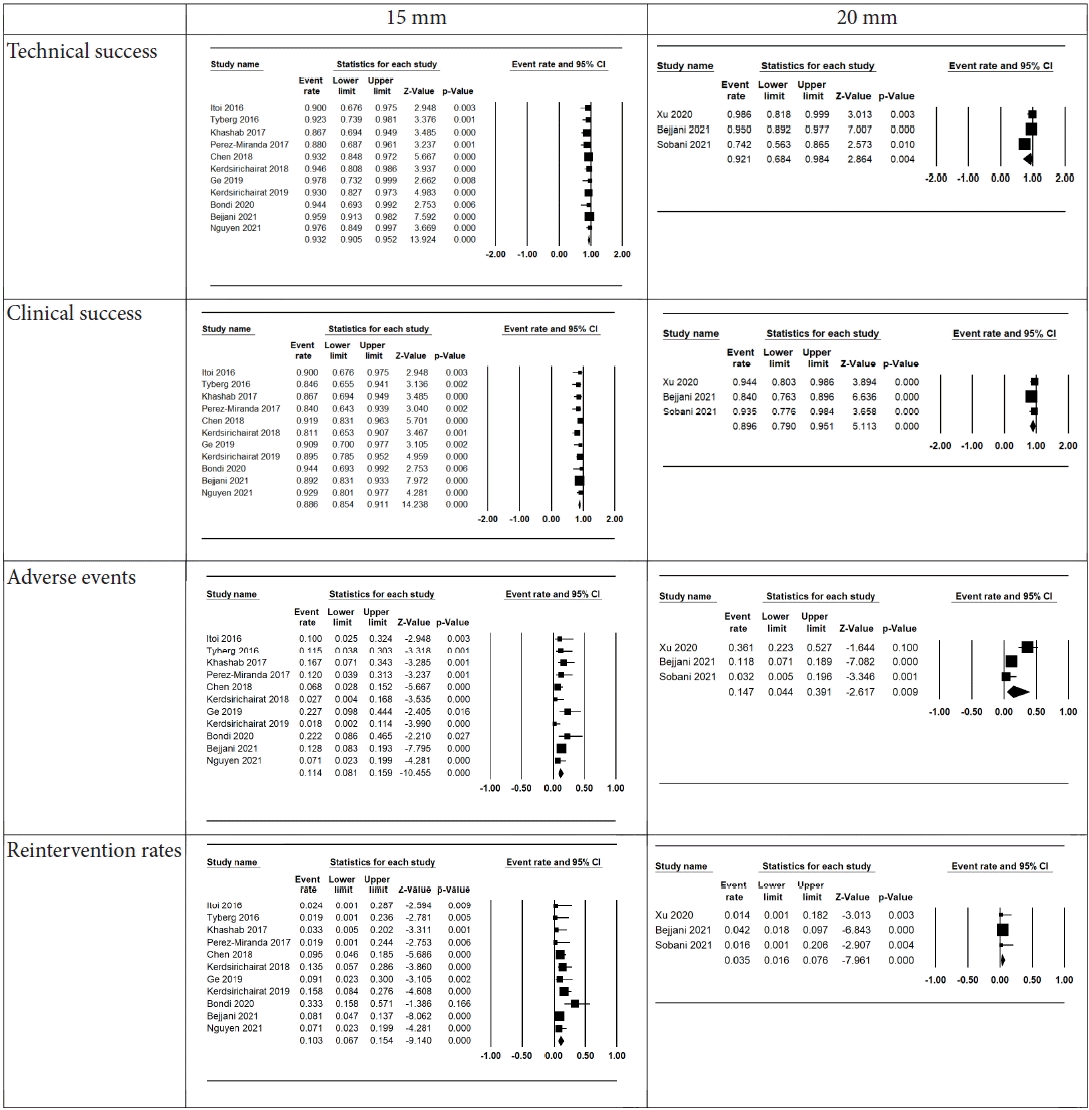
Table 1.
Characteristics of the included studies
| Study | Country | Study size (n) | Design | Benign cases (n) | Malignant cases (n) | Male (n) | Mean/median age (yr) | Technical success (n) | Clinical success (n) | Adverse event (n) | Reintervention (n) | Quality: NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies using 15×10 mm LAMS | ||||||||||||
| Tyberg et al. (2016)18 | USA | 26 | MRC | 9 | 17 | 11 | 66.2 | 24 | 22 | 3 | 0 | 6 |
| Itoi et al. (2016)19 | Japan | 20 | PC | 0 | 20 | 10 | 68 | 18 | 18 | 2 | 0 | 5 |
| Khashab et al. (2017)20 | USA | 30 | MRC | 0 | 30 | 17 | 70 | 26 | 26 | 5 | 1 | 7 |
| Perez-Miranda et al. (2017)21 | Spain | 30 | MRC | 8 | 17 | 11 | 63.9 | 22 | 21 | 3 | 0 | 5 |
| Kerdsirichairat et al. (2018)22 | USA | 37 | RC | 7 | 30 | 18 | 63 | 35 | 30 | 1 | 5 | 5 |
| Chen et al. (2018)23 | USA | 74 | MRC | 25 | 49 | 33 | 63 | 69 | 68 | 5 | 7 | 7 |
| Ge et al. (2019)10 | USA | 22 | RC | 0 | 22 | 12 | 66 | 22 | 20 | 5 | 2 | 7 |
| Kerdsirichairat et al. (2019)24 | USA | 57 | MRC | 9 | 48 | 28 | 65 | 53 | 51 | 1 | 9 | 6 |
| Bondi et al. (2020)25 | USA | 18 | RC | 0 | 18 | 8 | 64 | 17 | 17 | 4 | 6 | 6 |
| Nguyen et al. (2021)26 | USA | 41 | RC | 5 | 37 | 23 | 73 | 41 | 39 | 3 | 3 | 5 |
| Bejjani et al. (2022)27,a) | USA | 267a) | MRC | 0 | 148 | 86 | 68 | 142 | 132 | 19 | 12 | 6 |
| Studies using 20×10 mm LAMS | ||||||||||||
| Xu et al. (2020)28 | China | 36 | RC | 0 | 36 | 17 | 69 | 36 | 34 | 13 | 0 | 5 |
| Bejjani et al. (2022)27,a) | USA | 267a) | MRC | 0 | 119 | 66 | 67 | 113 | 100 | 14 | 5 | 6 |
| Sobani et al. (2021)14 | USA | 31 | PC | 8 | 23 | 17 | 61 | 31 | 29 | 1 | 0 | 5 |
Table 2.
Comparison of pooled technical success, clinical success, adverse event, and reintervention event rates
| 15 mm stent (n=499) | 20 mm stent (n=186) | p-value | |
|---|---|---|---|
| Technical success rate | 469 (93.2) | 172 (92.1) | 0.47 |
| Clinical success rate | 444 (88.6) | 163 (89.6) | 0.62 |
| Adverse event rate | 51 (11.4) | 28 (14.7) | 0.08 |
| Reintervention event rate | 45 (10.3) | 5 (3.5) | 0.008a) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download