Abstract
Objective
Deciphering the anatomy of posterior communicating artery (PCoA) aneurysms in relation to surrounding structures is essential to determine adjuvant surgical procedures. However, it is difficult to predict surgical structures through preoperative imaging studies. We aimed to present anatomical structures using preoperative high-resolution three-dimensional proton densityweighted turbo spin-echo magnetic resonance (PDMR) imaging with simple classification.
Methods
From January 2020 to April 2022, 30 patients underwent PDMR before microsurgical clipping for unruptured PCoA aneurysms in a single tertiary institute. We retrospectively reviewed the radiographic images and operative data of these patients. The structural relationship described by PDMR and intraoperative findings were compared. Subsequently, we classified aneurysms into two groups and analyzed the rate of adjuvant surgical procedures and contact with the surrounding structures.
Results
Correlations between preoperative PDMR predictions and actual intraoperative findings for PCoA aneurysm contact to the oculomotor nerve, temporal uncus, and anterior petroclinoid fold (APCF) reported a diagnostic accuracy of 0.90, 0.87, and 0.90, respectively. In 12 patients (40.0%), an aneurysm dome was located on the plane of the oculomotor triangle and was classified as the infratentorial type. Compared to the supratentorial type PCoA aneurysm, adjuvant procedures were required more frequently (66.7% vs. 22.2%, p=0.024) for infratentorial type PCoA aneurysm clipping.
Posterior communicating artery (PCoA) aneurysms account for 15–25% of all intracranial aneurysms [18]. Preoperative prediction of the surrounding soft tissue structures such as the cavernous sinus roof, oculomotor nerve, and medial aspect of the temporal lobe (uncus) is crucial to avoid surgical complications. Moreover, it is helpful to prepare for additional surgical procedures (e.g., anterior clinoidectomy, tentorial incision) before commencing the surgery [3].
Previous reports have attempted to predict the anatomical relationship of PCoA aneurysms to surrounding structures and predict additional procedures preoperatively [5,14,16]. These reports presented the angles of the internal carotid artery (ICA) or distance from a certain point of the anterior clinoid process (ACP) to the proximal aneurysm neck, and attempted to estimate the necessity of anterior clinoidectomy with incision of the anterior petroclinoid fold (APCF) [5,16]. However, these studies used an indirect method of estimating the positional relationship by calculating the value from computed tomography angiography (CTA) or transfemoral catheter angiography (TFCA) images.
Proton density-weighted turbo spin-echo magnetic resonance (PDMR) is a basic spin-echo pulse sequence generated from a long repetition time and a short echo time. It shows high resolution and excellent signal distinction of vessels containing cerebrospinal fluid (CSF) and other neural structures. Some studies employed PDMR for the differential diagnosis of PCoA aneurysms with the infundibulum, and reported the radiological accuracy of PDMR for the diagnosis of aneurysms in the circle of Willis and arterial dissection [8,9,18]. Furthermore, its high-resolution power helps to distinguish the location of an aneurysm from the thin distal dural ring (DDR) and visualize culprit perforators [12,19].
Therefore, we aimed to present the predictive value of PDMR for the relationship between PCoA aneurysms and the surrounding soft tissue structures. We also suggest a simple classification of PCoA aneurysms based on their relationship with the APCF and oculomotor triangle using PDMR. The classification can be used to evaluate the surgical complexity preoperatively.
This study was approved by Yonsei University Institutional Review Board (IRB), which waived the requirement for informed consent, given the retrospective study design (IRB No. 4-2022-0566). In this retrospective study, we reviewed 84 patients who underwent microsurgical clipping for PCoA aneurysms between January 2020 and April 2022. Among them, 30 patients underwent PDMR within a month prior to surgery for surgical anatomy evaluation. All patients were diagnosed with unruptured PCoA aneurysms, without cranial nerve symptoms, or other neurological signs.
PDMR was performed using a 3.0 T MRI system (Ingenia CX; Philips Medical Systems, Best, Netherlands) with a 32-channel head coil. The scanning parameters for PDMR were as follows : repetition time, 1800 ms; echo time, 45 ms; field of view, 200×200 mm; matrix, 448×428; slice thickness, 0.4 mm (interpolated to 0.2 mm); scan coverage, 3 cm; turbo spin-echo factor, 80; sensitivity encoding factor, 2.5×1.5; number of excitations, 1; and scan time, 5 minutes 20 seconds.
After acquisition of PDMR images, post-processing was performed using a commercial 3D image processing software (Aquarius iNtuition version 4.4.12; TeraRecon 3D workstation, San Mateo, CA, USA) to attain the precise discrimination between aneurysms and the surrounding soft tissue structures.
To quantify the predictive accuracy of PDMR, we set three specific anatomical points as the landmarks (the oculomotor nerve, temporal uncus, and the APCF medial margin) to demarcate the region of interest. Three neurosurgeons, excluding the operator of each case, predicted contact between these structures and the aneurysm using PDMR, and confirmed actual surgical findings based on the operative records. In case of any disagreement, consensus was reached through discussion.
We classified PCoA aneurysms into two types according to the aneurysmal position relative to the level of the APCF and oculomotor triangle. When the basement of the aneurysm contour projected below the level of the APCF on the coronal section of PDMR or below the space occupied by the oculomotor triangle in the axial section of PDMR, it was defined as an “infratentorial” aneurysm and otherwise, as a “supratentorial” aneurysm.
We compared the differences in complexities encountered during surgeries between the two types of aneurysms. The spatial relationships with other structures that needed attention and the frequency of additional intraoperative procedures (e.g., anterior clinoidectomy, subpial dissection, and dural fold resection) were reviewed.
The descriptive statistics were presented as numbers and percentages for categorical variables and means with standard deviations for continuous variables. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for concurrence between PDMR prediction and intraoperative findings were calculated. The predictive values were analyzed using pooled data from three observers. Analysis of variance and Fisher’s exact test were used to compare the characteristics and outcomes between the two PCoA aneurysm groups. All statistical analyses were performed using the SPSS statistical software for Windows, version 26 (IBM Corp., Armonk, NY, USA). Two-sided p-value less than 0.05 was considered as statistically significant.
A total of 30 patients (mean±standard deviation age, 63.73±7.28 years; four males and 26 females) with PCoA aneurysms were assessed. The maximum diameter of the aneurysms ranged from 2.24–20.97 mm (6.22±3.79 mm). The mean ASPECT ratio and Dome-to-Neck ratio were 1.12 and 1.37, respectively. Aneurysm projections were divided into lateral and posterior projections in six and 24 cases, respectively [1]. In addition, there were 11 cases (36.7%) with fetal-type posterior cerebral artery (PCA) (Table 1).
We analyzed the predictive power of PDMR for aneurysmal contact with the oculomotor nerve, temporal uncus, and APCF and compared it with intraoperative findings. There were 14, eight, and 20 cases of positive findings on PDMR, respectively, and 12, five, and 19 cases were confirmed to be concurrent with the surgical findings, respectively.
The correlation between preoperative PDMR and intraoperative findings for aneurysmal contact to the oculomotor nerve, temporal uncus, and APCF had sensitivity values of 0.92, 0.83, and 0.90, and specificity values of 0.88, 0.88, and 0.89, respectively. Moreover, positive predictive values of 0.86, 0.63, and 0.95, and negative predictive values of 0.94, 0.95, and 0.80, respectively, were obtained (Table 2).
There were 12 cases of infratentorial aneurysms and 18 cases of supratentorial aneurysms, with no significant differences in the mean aneurysmal size (5.46±2.41 mm vs. 6.73±4.48 mm, p=0.371) and other aneurysmal characteristics between the two groups.
We also inspected the frequency of aneurysm contact with the oculomotor nerve according to the aneurysm classification based on PDMR. The oculomotor nerve contact with the aneurysm dome was more frequently observed in infratentorial aneurysms than that in supratentorial aneurysms (91.7% vs. 11.1%, p=0.000). Aneurysmal contact with APCF showed a more frequent trend in the infratentorial type compared to the supratentorial type, but no statistical significance was observed (p=0.057).
There were significant differences between the two types of aneurysms in terms of the requirement for adjuvant surgical procedures, including ACP removal, resection of APCF, and subpial dissection of the temporal uncus (p=0.024). Eight (66.7%) out of 12 cases of infratentorial type PCoA aneurysms required an adjuvant surgical procedure during the surgery compared to that in four (22.2%) out of 18 cases of supratentorial aneurysms. However, according to the subcategorization, only ACP removal during surgery showed significant differences between the two groups of aneurysms (p=0.009) (Table 3).
A 63-year-old female patient with an incidental finding of a left unruptured PCoA aneurysm underwent surgical clipping. In PDMR images, the fundus of the aneurysm dome was located under the level of APCF (infratentorial type), with adhesion to its medial edge. Also, compression of the oculomotor nerve to the lateral side of the oculomotor triangle by the aneurysm was identified (Fig. 1A and B).
During the operation, we confirmed that the aneurysm dome was under APCF, which made it mostly invisible at initial exposure. After ACP removal and resection of APCF, there was a deviated oculomotor nerve attached right next to the aneurysm. This was consistent with the preoperative predictions. In the present case, anterior clinoidectomy was performed during the operation for aneurysm inspection and temporary clip application, as we had predicted based on the preoperative PDMR (Fig. 1C and D).
A 66-year-old male patient with an incidental finding of a right unruptured PCoA aneurysm underwent surgical clipping. PDMR confirmed that the basement of the aneurysm was at the level of the APCF and thus, this case was defined as the supratentorial type. Moreover, we derived further information from the preoperative PDMR. The absence of CSF spaces between the aneurysm and both the dural fold and temporal uncus could be indicative of a thick adhesion between these tissues, a condition that strongly predicts the need for additional surgical procedures (Fig. 2A).
Intraoperative findings revealed a thick adhesion of the aneurysm to the dural fold and temporal uncus that required subpial dissection of the temporal lobe and resection of the APCF to free the aneurysm, as predicted. There was no relationship between the oculomotor triangle or the oculomotor nerve itself (Fig. 2B-D).
A 57-year-old woman presented with a complaints of headache was diagnosed with unruptured left PCoA and ICA bifurcation aneurysms through brain magnetic resonance angiography (MRA). PDMR confirmed that the aneurysm dome projected to the lateral side of APCF outside of the oculomotor triangle. The absence of CSF signal between aneurysm and temporal lobe suggested there was adhesion between them (Fig. 3A and B).
Intraoperative findings confirmed that the aneurysm dome adhered to the temporal lobe, and the bottom side of the aneurysm was raised by the cavernous sinus lateral wall. There were no hidden structures under the dural fold, making aneurysmal neck manipulation and clipping relatively easy (Fig. 3C).
Understanding the soft tissue anatomy around PCoA aneurysms is important to avoid complications from microsurgical clipping. Some studies have reported surgical complications (e.g., oculomotor nerve palsy and premature rupture of the aneurysm) based on the contact between aneurysms and their surrounding structures [4,6].
Compared to CTA and TFCA, PDMR allows enhanced visualization of aneurysms and surrounding soft tissues with high resolution and excellent signal distinction. Therefore, it has been used in the neurovascular field and has proven to be useful in the diagnosis and treatment planning of neurovascular diseases. Previous studies have reported the diagnostic accuracy of PDMR as compared to time-of-flight MRA in distinguishing junctional dilatation and identifying true saccular aneurysms in the circle of Willis [9,18]. Additionally, Yoon et al. [19] localized paraclinoid aneurysms in relation to the DDR based on the signal distinction of CSF against the vessel and surrounding soft tissue structures. Furthermore, we suggest that these advantages of PDMR can be helpful for the prediction of surgical anatomy and planning for adjuvant surgical procedures during the clipping.
Several studies have focused on operative techniques for PCoA aneurysms [2,10,17]. In addition, they have reported preoperative prediction of anterior clinoidectomy or adjuvant APCF resection for better visualization of aneurysms [5,7,13,15,16]. Some studies have reported factors supporting the preoperative prediction of anterior clinoidectomy using TFCA and CTA [5,14,16]. Images acquired from these modalities have low prediction power for soft tissues; therefore, the authors conducted additional processes for indirect measurements and predictions. We intuitively visualized the relationships of certain adjacent structures to PCoA aneurysms and collected information from preoperative PDMR to simulate the intraoperative fields.
Park et al. [16] reported that measurement of the distal ICA angle on TFCA images can help predict the necessity of ACP removal before an operation. Kamide et al. [5] reported that the distances from the proximal neck of an aneurysm to both the ACP tip and ACP line on CTA can help predict the requirement of anterior clinoidectomy before an operation. These previous studies have presented good predictive values for anterior clinoidectomy. However, they did not analyze the soft tissues around the aneurysm or used complex mathematical calculations to measure the predictive values from the images. We focused on the identification and visualization of aneurysmal relationships with its surroundings in an intuitive manner.
Anatomical and clinical studies have classified PCoA aneurysms into three types (supratentorial, tentorial, and infratentorial) according to their relationship with APCF [3,11]. However, we tried to dichotomize PCoA aneurysms into two types (supratentorial and infratentorial) because there was low incidence of the tentorial type in a previous report, and we wanted to provide a more simple and clear classification system for immediate surgical application.
In cases of the infratentorial type, the aneurysms occupy the oculomotor triangle spaces that contain the oculomotor nerve passing through the cavernous sinus roof. This results in close location of the aneurysm to the oculomotor nerve and APCF. These characteristics limit aneurysmal manipulation during clip placements and require additional surgical procedures more frequently. In contrast, in cases of supratentorial aneurysms, wherein identification of the contour of the aneurysm and proximal parent artery is relatively easy in the surgical field, surgery can be performed with simple intradural manipulation. As a result, the present study suggests that by simply classifying aneurysms into two types, adjuvant surgical procedures and close relationships to neural structures could be anticipated. This simple classification of aneurysms did not show an exact prediction of the required procedure or structural relationships; however, this classification might be helpful in preoperative planning for clipping procedures.
The strength of the present study is that intuitive predictions and virtual reconstructions of surgical fields are available using PDMR findings without any supplementary mathematical calculations. We also reported that preoperative predictions using PDMR for surgical anatomies and spatial relationships were matched. This result supports that the predictive power of PDMR on PCoA aneurysms is valuable.
The present study has some limitations. It had a retrospective observational design and included patients from a single institution only. Moreover, the statistical power of the study is low owing to the limited number of samples. PDMR was not acquired from all consecutive patients who underwent clipping of PCoA aneurysms. Because of the natural limitations of a retrospective study design, the decisions for adjuvant surgical procedures were made intraoperatively and not based on PDMR categorizations. Therefore, prospective studies are required to confirm the predictive power of PDMR.
PDMR allows preoperative prediction of the positional relationship between PCoA aneurysms and surrounding soft tissues, and shows strong agreement with actual surgical findings. PDMR derived classification of PCoA aneurysms might provide useful information merely by allowing surgeons to intuitively review preoperative images without measuring any parameters.
Notes
ACKNOWLEDGMENTS
The 62th Annual Meeting of the Korean Neurosurgical Society, Oct 6th, 2022, Songdo Convensia, Incheon, Korea.
References
1. Fukuda H, Hayashi K, Yoshino K, Koyama T, Lo B, Kurosaki Y, et al. Impact of aneurysm projection on intraoperative complications during surgical clipping of ruptured posterior communicating artery aneurysms. Neurosurgery. 78:381–390. discussion 390. 2016.

2. Golshani K, Ferrell A, Zomorodi A, Smith TP, Britz GW. A review of the management of posterior communicating artery aneurysms in the modern era. Surg Neurol Int. 1:88. 2010.

3. González-Darder JM, Quilis-Quesada V, Talamantes-Escribá F, Botella-Maciá L, Verdú-López F. Microsurgical relations between internal carotid artery-posterior communicating artery (ICA-PComA) segment aneurysms and skull base: an anatomoclinical study. J Neurol Surg B Skull Base. 73:337–341. 2012.

4. Houkin K, Kuroda S, Takahashi A, Takikawa S, Ishikawa T, Yoshimoto T, et al. Intra-operative premature rupture of the cerebral aneurysms. Analysis of the causes and management. Acta Neurochir (Wien). 141:1255–1263. 1999.

5. Kamide T, Burkhardt JK, Tabani H, Safaee MM, Lawton MT. Preoperative prediction of the necessity for anterior clinoidectomy during microsurgical clipping of ruptured posterior communicating artery aneurysms. World Neurosurg. 109:e493–e501. 2018.

6. Kim E. Clip compression injury of the oculomotor nerve: its prevention and recovery. Korean J Neurotrauma. 16:85–89. 2020.

7. Kim JH, Kim JM, Cheong JH, Bak KH, Kim CH. Simple anterior petroclinoid fold resection in the treatment of low-lying internal carotid-posterior communicating artery aneurysms. Surg Neurol. 72:142–145. 2009.

8. Kim JW, Shin NY, Kim YD, Lee SK, Lim SM, Oh SW. Added value of 3D proton-density weighted images in diagnosis of intracranial arterial dissection. PLoS One. 11:e0166929. 2016.

9. Kim S, Chung J, Cha J, Kim BM, Kim DJ, Kim YB, et al. Usefulness of high-resolution three-dimensional proton density-weighted turbo spinecho MRI in distinguishing a junctional dilatation from an intracranial aneurysm of the posterior communicating artery: a pilot study. J Neurointerv Surg. 12:315–319. 2020.

10. Kuzmik GA, Bulsara KR. Microsurgical clipping of true posterior communicating artery aneurysms. Acta Neurochir (Wien). 154:1707–1710. 2012.

11. Lawton MT. Seven Aneurysms: Tenets and Techniques for Clipping. New York: Thieme;2011.
12. Lee SH, Jung SC, Kang DW, Kwon SU, Kim JS. Visualization of culprit perforators in anterolateral pontine infarction: high-resolution magnetic resonance imaging study. Eur Neurol. 78:229–233. 2017.

13. Matano F, Murai Y, Mizunari T, Yamaguchi M, Yamada T, Baba E, et al. Incision of the anterior petroclinoidal fold during clipping for securing the proximal space of an internal carotid artery-posterior communicating artery aneurysm: a technical note. Neurosurg Rev. 42:777–781. 2019.

14. Niibo T, Takizawa K, Sakurai J, Takebayashi S, Koizumi H, Kobayashi T, et al. Prediction of the difficulty of proximal vascular control using 3D-CTA for the surgical clipping of internal carotid artery-posterior communicating artery aneurysms. J Neurosurg. 134:1165–1172. 2020.

15. Nossek E, Setton A, Dehdashti AR, Chalif DJ. Anterior petroclinoid fold fenestration: an adjunct to clipping of postero-laterally projecting posterior communicating aneurysms. Neurosurg Rev. 37:637–641. 2014.

16. Park SK, Shin YS, Lim YC, Chung J. Preoperative predictive value of the necessity for anterior clinoidectomy in posterior communicating artery aneurysm clipping. Neurosurgery. 65:281–285. discussion 285-286. 2009.

17. Sanai N, Caldwell N, Englot DJ, Lawton MT. Advanced technical skills are required for microsurgical clipping of posterior communicating artery aneurysms in the endovascular era. Neurosurgery. 71:285–294. discussion 294-295. 2012.

18. Yim Y, Jung SC, Kim JY, Kim SO, Kim BJ, Lee DH, et al. Added diagnostic values of three-dimensional high-resolution proton density-weighted magnetic resonance imaging for unruptured intracranial aneurysms in the circle-of-Willis: comparison with time-of-flight magnetic resonance angiography. PLoS One. 15:e0243235. 2020.

Fig. 1.
A : Aneurysm (asterisk) located under the level of the APCF (white arrowhead). B : Laterally deviated oculomotor nerve (white arrow) with PCoA aneurysm of the affected side, contralateral normal oculomotor triangle (white triangle). Intraoperative photography, (C) PCoA aneurysm under the APCF (black dashed line) and (D) oculomotor nerve (black arrow) adjacent to PCoA aneurysm and concomitant anterior choroidal aneurysm. APCF : anterior petroclinoid fold, PCoA : posterior communicating artery
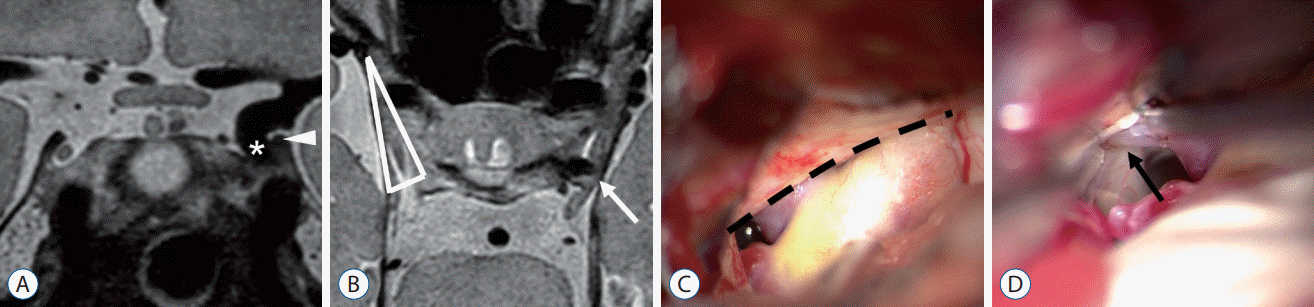
Fig. 2.
A : Coronal section of Rt. posterior communicating artery aneurysm on PDMR; aneurysm contact with temporal uncus (black arrow) and the APCF (white arrow) are indicated. Oculomotor nerve (white arrowhead). Intraoperative photographs, (B) thick adhesion of aneurysm to temporal uncus (black arrow), and (C) after subpial dissection of aneurysm from temporal uncus, adhesion to the APCF is noted. (D) After resection of the APCF to free the aneurysm, the oculomotor nerve (white arrow) below the APCF was noted. Rt. : right, PDMR : proton density-weighted turbo spin-echo magnetic resonance, APCF : anterior petroclinoid fold.
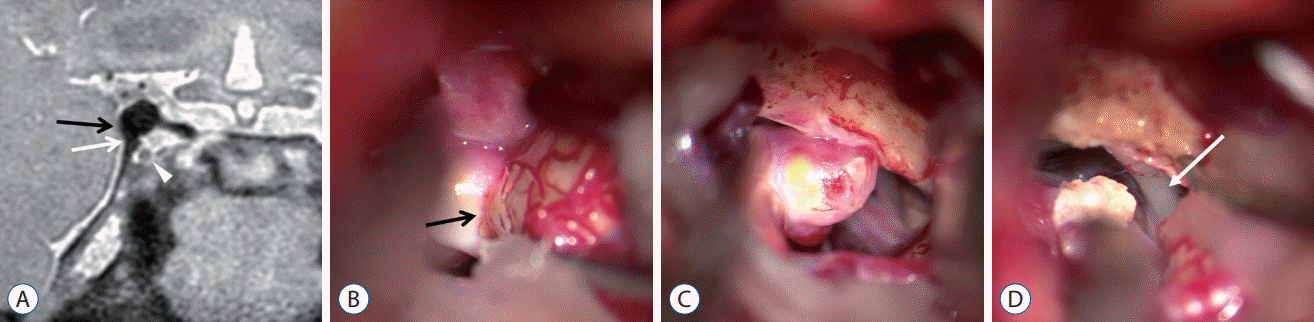
Fig. 3.
A and B : Lt. PCoA aneurysm (asterisk) caught between APCF and temporal uncus is shown on PDMR coronal and axial planes. Intraoperative photography, (C) Lt. PCoA aneurysms are caught between the APCF (white dashed line) and temporal uncus (T). Lt. : left, PCoA : posterior communicating artery, APCF : anterior petroclinoid fold, PDMR : proton density-weighted turbo spin-echo magnetic resonance.

Table 1.
Characteristics of patients who underwent microsurgical clipping for PCoA aneurysms
Table 2.
Accuracy of PDMR prediction for contact with surrounding structures and comparisons to the actual intraoperative findings
| TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|
| Oculomotor nerve | 12 | 15 | 2 | 1 | 0.92 | 0.88 | 0.86 | 0.94 | 0.90 |
| Temporal uncus | 5 | 21 | 3 | 1 | 0.83 | 0.88 | 0.63 | 0.95 | 0.87 |
| APCF | 19 | 8 | 1 | 2 | 0.90 | 0.89 | 0.95 | 0.80 | 0.90 |
Table 3.
Comparison of the characteristics of infratentorial and supratentorial type PCoA aneurysms




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download