Abstract
Objective
Preoperative transarterial embolization (TAE) of tumor feeders in hypervascular spine metastasis is known to reduce intraoperative estimated blood loss (EBL) during surgery. The effect of TAE varies for several reasons, and one controllable factor is the timing between embolization and surgery. However, the adequate timing remains unclear. This study aimed to evaluate the timing and other factors that reduce EBL in spinal metastasis surgery through a meta-analysis.
Methods
A comprehensive database search was performed to identify direct comparative studies of EBL stratified by the timing of surgery after TAE for spinal metastasis. EBL was analyzed according to the timing of surgery and other factors. Subgroup analyses were also performed. The difference in EBL was calculated as the mean difference (MD) and 95% confidence interval (CI).
Results
Among seven studies, 196 and 194 patients underwent early and late surgery after TAE, respectively. The early surgery was defined as within 1–2 days after TAE, while the late surgery group received surgery later. Overall, the MD in EBL was not different according to the timing of surgery (MD, 86.3 mL; 95% CI, -95.5 to 268.1 mL; p=0.35). A subgroup analysis of the complete embolization group demonstrated that patients who underwent early surgery within 24 hours after TAE had significantly less bleeding (MD, 233.3 mL; 95% CI, 76.0 to 390.5 mL; p=0.004). In cases of partial embolization, EBL was not significantly different regardless of the time interval.
In surgery for hypervascular metastatic spinal tumors, massive intraoperative bleeding may make major complications and safe operative resection technically challenging [2,5,8,16]. Preoperative transarterial embolization (TAE) of such tumors is a preemptive method to reduce intraoperative bleeding, and improve the surgeon’s ability to safely perform surgery, and maximize tumor resection [1,6,17,19]. TAE was first reported by Feldman et al. [3] in 1975. Gelatin products have been used for neurovascular embolization for almost half a century, and recently embolic agents such as polyvinyl alcohol (PVA) particles, N-butyle-2-cyanoacrylate (NBCA), and Onyx [4].
Although preoperative TAE for hypervascular spinal metastasis has been established to lower intraoperative estimated blood loss (EBL), even in embolized patients significant blood loss may occur [21]. Some studies reported that preoperative TAE significantly reduced intraoperative EBL [10,25]. Conversely, other studies reported that EBL did not differ regardless of TAE [14,16]. These inconsistent results reflect the difficulty of accurately assessing factors that contribute to EBL in embolized versus non-embolized tumors [15,18]. The efficacy of embolization is affected by the tumor (histology and tumor volume), the patient (age/sex and obesity), and the physician (degree of embolization and time interval to surgery) [11-13,23].
Among the factors affecting efficacy of TAE, the time interval between TAE and surgery has been discussed as a controllable factor that might impact the efficacy of the procedure. Heran [7] reported that surgery done within 72 hours of embolization (optimally within 24 hours) decreased perioperative blood loss compared with surgery done beyond this period. A previous paper reported that the EBL was found to be significantly lower in patients who had surgery within 1 day after TAE than in those who had surgery performed the following day [9]. Another paper objected to the results of that study [16]. Other researchers demonstrated no significant difference in EBL between patients who underwent immediate and delayed surgery after TAE [16,20,22]. Therefore, there is no clear evidence regarding the appropriate timing of surgery after TAE.
The aim of this study was to analyze the effect of the time interval between TAE and surgery for spinal metastasis on EBL and to identify other controllable factors through a systematic review and meta-analysis.
Institutional Review Board review and approval was not required for this type of study.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we performed a meta-analysis of clinical studies that compared the timing of surgery after preoperative tumor embolization. A systematic search of PubMed, EMBASE, Web of Science, and the Cochrane Database was conducted on December 23, 2021, independently by two separate reviewers (W.T.Y. and C.H.L.). The search terms used were “spine” (or “spinal”) AND “metastasis” (or “metastases”) AND “embolization” AND “schedule” (or “timing”). We included only papers written in English, and only the largest study was included if there were overlapping study populations. After excluding duplicate articles, the search results were screened by title and abstracts for the following exclusion criteria : reviews, case reports, letters, experiments; no adjuvant TAE; and no tumor resection. After eliminating the excluded papers, full-text articles were obtained, and studies were thoroughly screened again using the same exclusion criteria. We excluded articles for the following reasons: no inclusion of spinal metastasis; a comparison of blood loss with and without TAE; and the use of another evaluation unit for blood loss (e.g., hemoglobin). We also examined the references of all included papers to find other relevant articles. Any inconsistencies between the two reviewers were resolved by discussion and agreement.
The goal of the search was to identify articles that met the following inclusion criteria : direct comparative studies of EBL stratified by the timing of surgery after TAE for spinal metastasis. Studies were included in the data extraction if they reported EBL in an early and late interval between TAE and surgery. When available, we recorded characteristics such as the number of patients, age, sex, country, study period, material of TAE, degree of TAE, and the definition of time interval. Outcomes were extracted from the texts and tables of the studies. If data from individual cases were available, we collected all data, including the time to surgery and EBL.
Quality assessment was done independently by a pair of authors (W.T.Y. and C.H.L.); any inconsistencies between them were resolved through discussion. Study quality was determined for controlled observational cohort studies with the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS). Summary estimates were evaluated using the calculated effect size based on the mean difference (MD) and 95% confidence intervals (CIs), using Review Manager (RevMan) version 5.4. The Cochrane Collaboration, 2020. The data from each included study were weighted by the inverse of the variance of the results, thereby accounting for both within- and between-study error. Heterogeneity was assessed in all meta-analyses using the Higgins I2 statistic and the Cochran Q test. According to the heterogeneity, a random-effect or fixed-effect model was chosen. We performed a subgroup analysis to examine whether the effects varied according to the degree of embolization. We assessed publication bias by visual inspection of funnel plots and by calculating the p-value (one-sided) for Egger’s intercept. All tests were two-sided, with p-values less than 0.05 deemed significant.
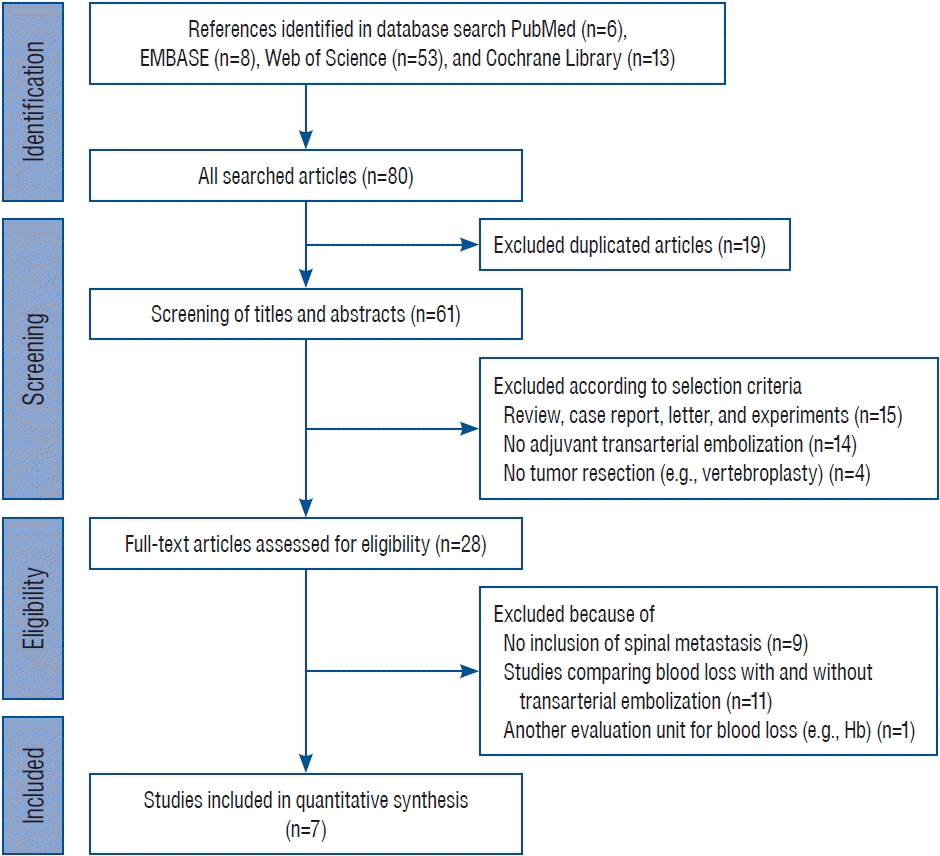
An initial literature search resulted in six papers in PubMed, eight in EMBASE, 53 in the Web of Science, and 13 in the Cochrane Library. Of these 80 studies, 19 were excluded as duplicates. After screening titles and abstracts, 33 papers were excluded, because 15 were reviews, case reports, letters, or experiments, 14 were not about adjuvant TAE, and four were studies without tumor resection. The 28 remaining studies were subjected to full-text review, resulting in the exclusion of 21 studies. The reasons for the exclusion of these articles were no inclusion of spinal metastasis (n=9), studies comparing EBL with and without TAE (n=11), and the use of another evaluation unit (hemoglobin) for blood loss (n=1). Finally, seven papers were included in this meta-analysis. The detailed results of the selection process are shown in Fig. 1.
Table 1 summarizes the characteristics of the studies included in this analysis, which contained 390 participants [9,10,14,20,21,23,24]. All seven studies were retrospective studies, and the study period was from 1999 to 2014. The degree of tumor embolization was divided into complete, partial, and/or near complete, and the definition of complete embolization was usually 90% (ranging from 75% to 90%). The reported rate of complete embolization was 49.5% to 92.8%. The most frequently used embolization material was PVA particles, followed in descending order by gelatin sponge, coil, and NBCA glue.
Two of the seven studies included both metastatic tumors (n=120) and primary bone tumors (n=90) [23,24]. This may have increased the heterogeneity of the study target. However, these two studies were finally enrolled in this meta-analysis because the patients with primary bone tumors were distributed evenly across each group, and both groups of tumors were diagnosed as hypervascular by the clinicians. The other five studies included only metastatic spine tumors.
The definition and frequency of complete embolization varied among the included studies. Four papers defined complete embolization as a 90% decrease of tumor blush [10,20,21,23], and two papers used thresholds of 80% and 75% [9,14]. One paper did not clearly describe the criterion that was used [24]. The frequency of complete embolization ranged from 30% to 93%.
Regarding the timing of surgery, the criteria used to distinguish between early surgery and late surgery were roughly 1–2 days after TAE. Three papers defined the early surgery as an operation within the same day after embolization; two papers defined it as an operation within 24 hours; one paper defined it as an operation within 2 days; and one paper defined it as an operation within 48 hours. In a subgroup analysis, we collected data from individual patients in which the timing of surgery after embolization and the EBL were recorded.
Among the 390 patients from seven studies, 196 patients underwent surgery early after TAE, and 194 patients had surgery later. The proportion of male patients was 60.3%, and the mean age at surgery was 56.8 years in the included studies. Six studies of 261 patients reported the exact histologic type of tumor, and renal cell carcinoma was the most common tumor pathology (n=104, 39.9%), followed by thyroid cancer (n=50, 19.2%). In all seven studies, there were a total of five reported complication cases : two cases of acute stroke and one case each of cord ischemia, severe hypertension, and transient visual field defect. All complications occurred within several hours after TAE.
In the overall comparison, the early surgery group underwent surgery within 1–2 days after TAE, and the late surgery group received surgery at least 1 day after TAE. All studies demonstrated a consistent tendency for less EBL in the early surgery group, but without statistical significance (MD, 86.3 mL; 95% CI, -95.5 to 268.1 mL; p=0.35) as shown in Fig. 2. We divided the results according to the timing of surgery (within 1 day/24 hours and within 2 days/48 hours), and neither group showed statistical significance. Five studies used within 1 day as the standard of timing, and the MD of EBL was 64.6 mL (95% CI, -122.9 to 252.1 mL; p=0.50) [9,10,14,20,23]. The others used 2 days, and the MD of EBL was 425.0 mL (95% CI, -315.4 to 1165.3 mL; p=0.26) [21,24].
We selected individual cases of complete embolization with a time interval of within 1 day in three studies [9,10,21]. In the data from individual participants who underwent complete embolization and surgery within 1 day after TAE, the early surgery group showed 248.7 mL (95% CI, 84.4 to 413.0 mL) less bleeding than the late surgery group. This difference was statistically significant (Fig. 3). One study that reported a 93% rate of complete embolization was also included in the subgroup analysis [23]. This analysis also showed a significant difference in EBL (MD, 233.3 mL; 95% CI, 76.0 to 390.5 mL; p=0.004), as presented in Fig. 3.
To clarify the effect of degree of TAE, we divided the data into complete and partial embolization. Seventy-three patients from three studies were described as undergoing complete embolization [10,20,21]. Overall, the complete embolization group showed significantly less EBL than the partial embolization group (Fig. 4), and the MD of EBL was 219.8 mL (95% CI, 39.3 to 400.2 mL; p=0.02). Among these patients, those who underwent early surgery showed a significant difference in EBL (MD, 326.7 mL; 95% CI, 73.9 to 579.5 mL; p=0.01), but a significant difference was not found in the late surgery group (MD, 134.7 mL; 95% CI, -133.6 to 403.0 mL; p=0.32).
Single elimination of each study did not affect the overall results of the meta-analysis. The Egger’s test result for the summary estimates of all papers was 0.29 (p=0.60) for EBL. The funnel plot was symmetric, suggesting an absence of significant publication bias within the studies. These results imply no substantial evidence of publication bias in the dataset.
The efficacy of TAE may be affected by some factors during surgery for hypervascular spinal metastasis, and the effect of time interval between TAE and surgery was analyzed in this study. Both complete embolization of feeding vessels and a short time interval (within 24 hours) between TAE and surgery were significantly associated with reduced EBL in patients with hypervascular spinal metastasis. In patients who underwent partial embolization or late surgery, EBL was not significantly different regardless of the time interval.
The duration of the effect of TAE is not clearly known. The main concern is arterial recanalization and collateral blood flow establishment [4,9]. In general, the sooner the excision is performed, the lower the degree of perioperative blood loss. A previous study had argued that early surgery was effective only if a temporary embolization agent such as gelfoam was used [16]. However, the majority of enrolled studies in this meta-analysis used PVA; the studies demonstrated a consistent tendency of less bleeding in the early surgery group, and the summary estimate of EBL showed a statistically significant difference. This means that the effect of embolic materials such as gelfoam, as well as PVA, may diminish within a day. Therefore, the optimal time for surgery would be immediately following the embolization, and spinal metastasis surgery is preferable to perform within 1 day after embolization if possible.
In cases of partial embolization, EBL was not different regardless of the time interval. One possible interpretation of this finding is that the partial embolization effect is maintained for several days, but it would be more reasonable to conclude that partial embolization may have little effect on reducing EBL considering the short-term effect of complete embolization. However, this finding needs to be interpreted with caution because we did not directly compare EBL between non-embolized and partial embolized tumors, and the degree of embolization was not included in the search keywords.
The finding that early surgery reduces EBL only with complete embolization may lead to confusion as to which is independent factor for reducing EBL : surgery timing, complete embolization, or both. To clarify it, a subgroup analysis was performed according to the degree of embolization. Overall, the complete embolization group demonstrated less bleeding than the partial embolization group, and early surgery did not show a substantial difference in bleeding. However, the combination of complete embolization followed by surgery within 1 day/24 hours showed a significant effect on reducing EBL. This means that both complete embolization and early surgery after TAE are essential to reduce EBL.
When discussing the optimal timing of surgery after embolization, it is necessary to emphasize that complete embolization must be performed first. If complete embolization is performed, surgery within 1 day or 24 hours after embolization would significantly reduce intraoperative bleeding in comparison to surgery performed after 24 hours. This does not mean that the optimal surgical timing is within exactly 1 day after TAE. Based on current evidence, surgery for spinal metastasis within 1 day after complete embolization may reduce EBL because the majority of studies used a cut-off value of within 1 day or 24 hours. Probably, the optimal timing of surgery may be the sooner the better.
Some limitations need to be acknowledged and addressed regarding the present study. First, there was substantial heterogeneity in primary cancer, vascularity, definition of complete embolization, and kinds of surgery. Research on spinal metastasis usually faces this problem. In this field, a metaanalysis may be a good method that can integrate data from multiple studies and reduce confounding variables. This study included only direct comparative studies in which factors other than the timing of surgery were controlled. Second, definitions of completeness of embolization vary among studies. In four out of seven studies [10,20,21,23], the definition of complete (or successful) embolization was 90%, while in two other studies, it varied from 75% to 80% [9,15]. The degree of embolization was evaluated by the amount of tumor blush reduction, which is determined subjectively by the operator. There is no objective and consistent tool for evaluating the degree of TAE. This may weaken the clinical effectiveness of this paper. Finally, we did not conduct a bottom-up analysis to determine the appropriate timing for surgery, but instead conducted an analysis based on the standards that previous papers used. The findings, therefore, must be interpreted with caution. A large-scale prospective study is needed to determine the optimal surgical timing after embolization.
Notes
References
1. Clausen C, Dahl B, Frevert SC, Hansen LV, Nielsen MB, Lönn L. Preoperative embolization in surgical treatment of spinal metastases: singleblind, randomized controlled clinical trial of efficacy in decreasing intraoperative blood loss. J Vasc Interv Radiol. 26:402–412.e1. 2015.
2. De la Garza Ramos R, Echt M, Benton JA, Gelfand Y, Longo M, Yanamadala V, et al. Accuracy of freehand versus navigated thoracolumbar pedicle screw placement in patients with metastatic tumors of the spine. J Korean Neurosurg Soc. 63:777–783. 2020.
3. Feldman F, Casarella WJ, Dick HM, Hollander BA. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 123:130–139. 1975.
4. Gottfried ON, Schloesser PE, Schmidt MH, Stevens EA. Embolization of metastatic spinal tumors. Neurosurg Clin N Am. 15:391–399. 2004.

5. Griessenauer CJ, Salem M, Hendrix P, Foreman PM, Ogilvy CS, Thomas AJ. Preoperative embolization of spinal tumors: a systematic review and meta-analysis. World Neurosurg. 87:362–371. 2016.

6. He Z, Wong ST, Yam KY. Newly-Diagnosed, histologically-confirmed central nervous system tumours in a regional hospital in hong kong : an epidemiological study of a 21-year period. J Korean Neurosurg Soc. 63:119–135. 2020.

7. Heran MK. Preoperative embolization of spinal metastatic disease: rationale and technical considerations. Semin Musculoskelet Radiol. 15:135–142. 2011.

8. Hong JT, Kim IS, Lee HJ, Park JH, Hur JW, Lee JB, et al. Evaluation and surgical planning for craniovertebral junction deformity. Neurospine. 17:554–567. 2020.

9. Kato S, Hozumi T, Takaki Y, Yamakawa K, Goto T, Kondo T. Optimal schedule of preoperative embolization for spinal metastasis surgery. Spine (Phila Pa 1976). 38:1964–1969. 2013.

10. Kato S, Murakami H, Minami T, Demura S, Yoshioka K, Matsui O, et al. Preoperative embolization significantly decreases intraoperative blood loss during palliative surgery for spinal metastasis. Orthopedics. 35:e1389–e1395. 2012.

12. Kim KR, Le Huec JC, Jang HJ, Noh SH, Park JY, Ha Y, et al. Which is more predictive value for mechanical complications: fixed thoracolumbar alignment (T1 pelvic angle) versus dynamic global balance parameter (odontoid-hip axis angle). Neurospine. 18:597–607. 2021.

13. Kobayashi K, Ozkan E, Tam A, Ensor J, Wallace MJ, Gupta S. Preoperative embolization of spinal tumors: variables affecting intraoperative blood loss after embolization. Acta Radiol. 53:935–942. 2012.

14. Kumar N, Tan B, Zaw AS, Khine HE, Maharajan K, Lau LL, et al. The role of preoperative vascular embolization in surgery for metastatic spinal tumours. Eur Spine J. 25:3962–3970. 2016.

15. Kumar N, Zaw AS, Khine HE, Maharajan K, Wai KL, Tan B, et al. Blood loss and transfusion requirements in metastatic spinal tumor surgery: evaluation of influencing factors. Ann Surg Oncol. 23:2079–2086. 2016.

16. Ma J, Tullius T, Van Ha TG. Update on preoperative embolization of bone metastases. Semin Intervent Radiol. 36:241–248. 2019.

17. Nagoshi N, Nori S, Tsuji O, Suzuki S, Okada E, Yagi M, et al. Surgical and functional outcomes of expansive open-door laminoplasty for patients with mild kyphotic cervical alignment. Neurospine. 18:749–757. 2021.

18. Park SJ, Lim SH, Kim YJ, Moon KS, Kim IY, Jung S, et al. The tumor control according to radiation dose of gamma knife radiosurgery for small and medium-sized brain metastases from non-small cell lung cancer. J Korean Neurosurg Soc. 64:983–994. 2021.

19. Perez-Roman RJ, McCarthy D, Luther EM, Lugo-Pico JG, Leon-Correa R, Gaztanaga W, et al. Effects of body mass index on perioperative outcomes in patients undergoing anterior cervical discectomy and fusion surgery. Neurospine. 18:79–86. 2021.

20. Quraishi NA, Purushothamdas S, Manoharan SR, Arealis G, Lenthall R, Grevitt MP. Outcome of embolised vascular metastatic renal cell tumours causing spinal cord compression. Eur Spine J. 22(Suppl 1):S27–S32. 2013.

21. Schmidt R, Rupp-Heim G, Dammann F, Ulrich C, Nothwang J. Surgical therapy of vertebral metastases. Are there predictive parameters for intraoperative excessive blood loss despite preoperative embolization? Tumori. 97:66–73. 2011.
22. Sun S, Lang EV. Bone metastases from renal cell carcinoma: preoperative embolization. J Vasc Interv Radiol. 9:263–269. 1998.

23. Tang B, Ji T, Guo W, Tang X, Jin L, Dong S, et al. Which is the better timing between embolization and surgery for hypervascular spinal tumors, the same day or the next day?: A retrospective comparative study. Medicine (Baltimore). 97:e10912. 2018.
24. Wilson MA, Cooke DL, Ghodke B, Mirza SK. Retrospective analysis of preoperative embolization of spinal tumors. AJNR Am J Neuroradiol. 31:656–660. 2010.
25. Wirbel RJ, Roth R, Schulte M, Kramann B, Mutschler W. Preoperative embolization in spinal and pelvic metastases. J Orthop Sci. 10:253–257. 2005.
Fig. 2.
A forest plot of intraoperative blood loss by surgery timing. The standard of early surgery after embolization was within 0 day or 24 hours in five studies and within 2 days or 48 hours in two studies. The early surgery group shows a trend for less bleeding, but the difference is not statistically significant. SD : standard deviation, IV : inverse variance, CI : confidence interval.
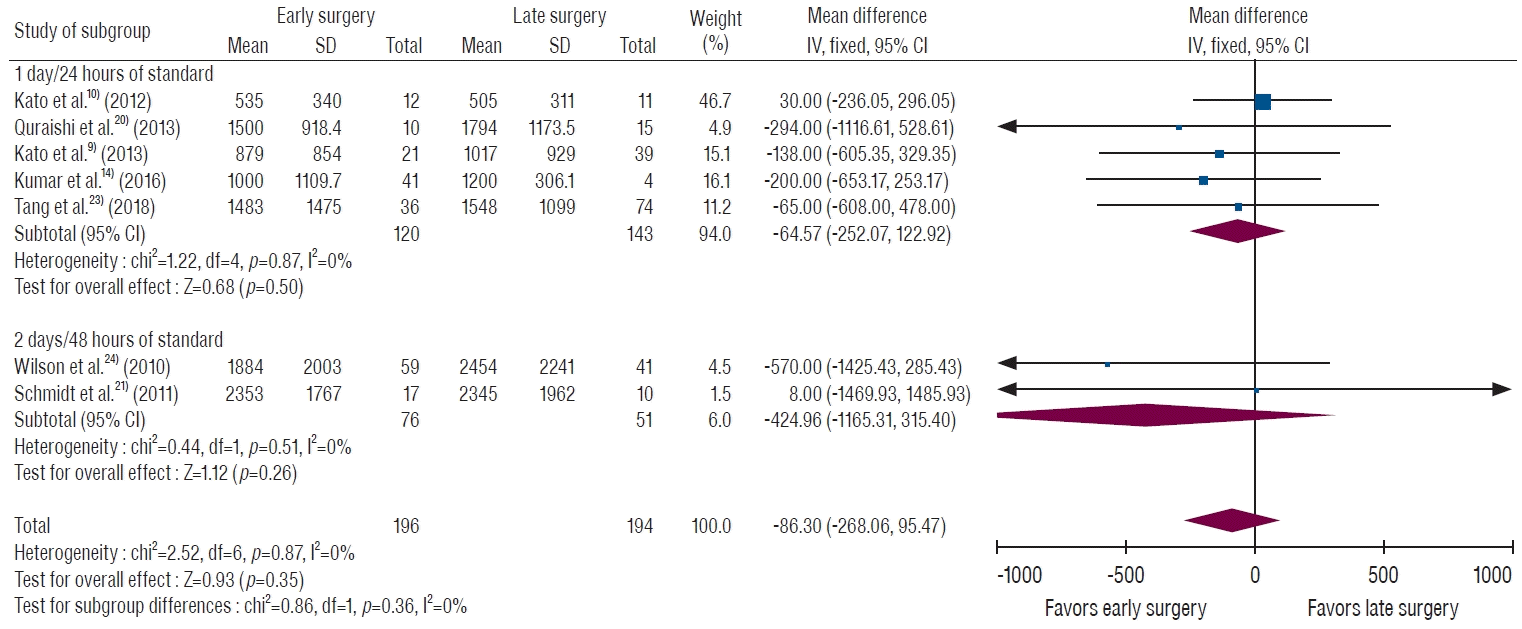
Fig. 3.
A forest plot of intraoperative blood loss by surgery timing (≤24 hours or not) in patients with complete embolization. Individual participant data from three studies showed significantly less bleeding in the early surgery group. When adding data from a study stating that complete embolization was achieved in 93% of patients, the early surgery group also shows significantly less bleeding than the late surgery group. SD : standard deviation, IV : inverse variance, CI : confidence interval.
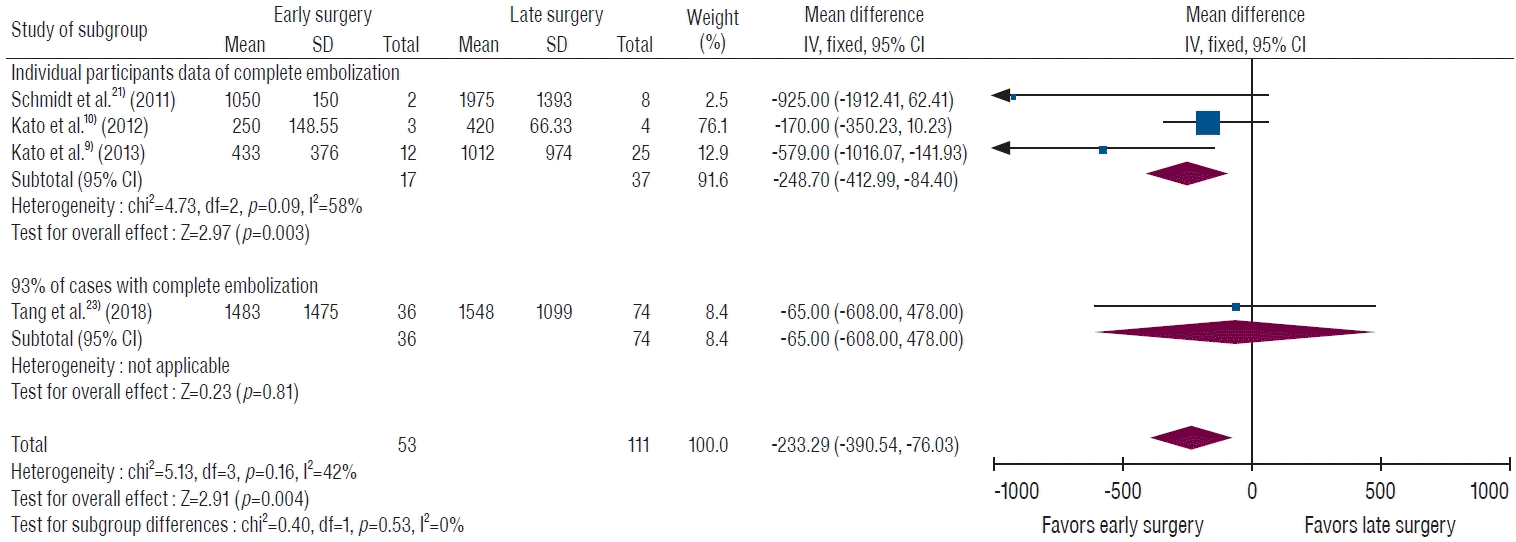
Fig. 4.
A forest plot of intraoperative blood loss by the degree of embolization stratified by surgery timing. The complete embolization group showed less bleeding than the partial embolization group. The combination of both complete embolization and early surgery was significantly associated with reduced blood loss. SD : standard deviation, IV : inverse variance, CI : confidence interval.
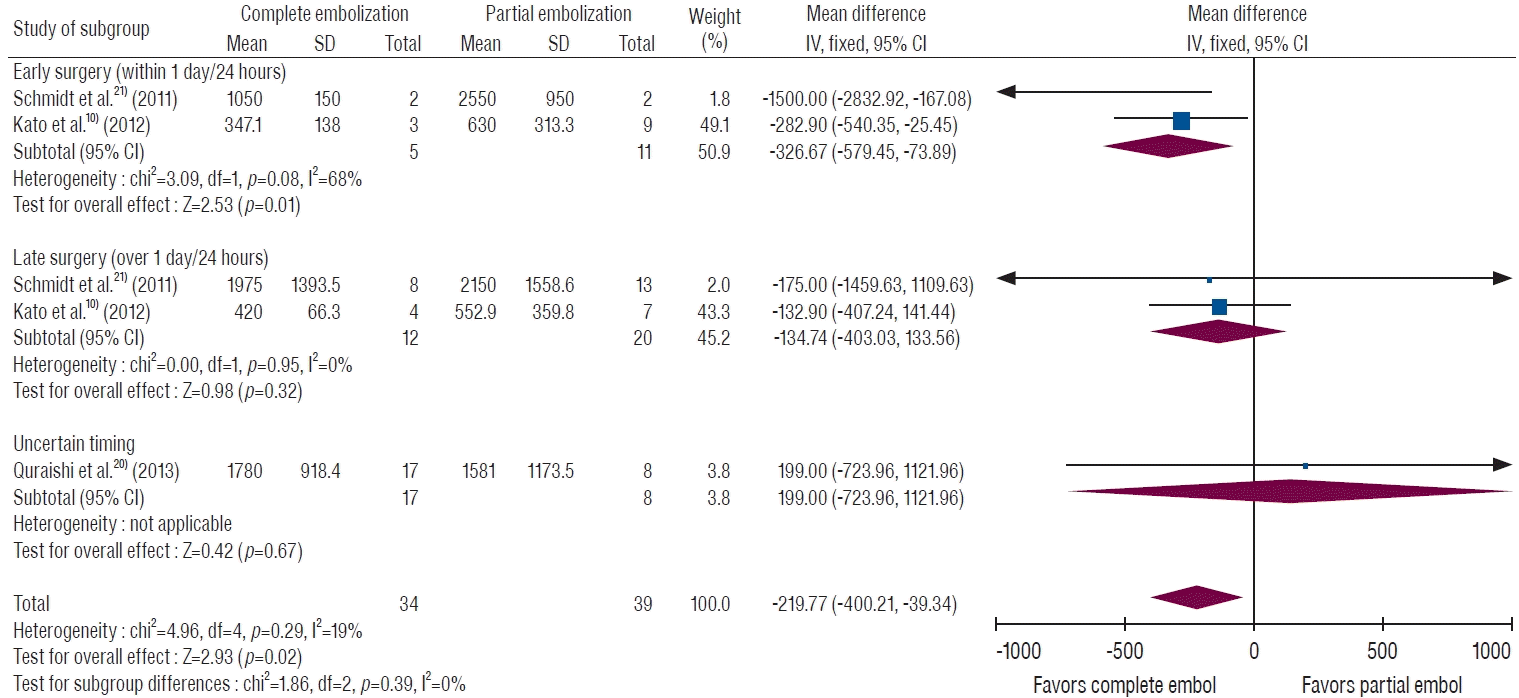
Table 1.
Baseline characteristics of included studies
| Study | Disease | Study period | Embolization materials | No. of patients (male %) | Mean age (years) | Degree of CE (%) | Rate of CE (%) | No. of complications |
|---|---|---|---|---|---|---|---|---|
| Wilson et al. [24] (2010) | Mets, PBT | 1999 to 2006 | PVA, coil, gelatinsponge | 100 (66.0) | 54 | ND | 49.5 | 1 |
| Schmidt et al. [21] (2011) | Mets | 2002 to 2008 | PVA, NBCA glue | 27 (63.0) | 67 | 90.0 | 37.0 | 0 |
| Kato et al. [10] (2012) | Mets | 2000 to 2010 | PVA, gelatin sponge | 23 (65.2) | 62.8 | 90.0 | 30.4 | 0 |
| Kato et al. [9] (2013) | Mets (RCC & thyroid) | 2004 to 2012 | Gelatin sponge | 60 (50.8) | 63.8 | 75.0 | 61.5 | 0 |
| Quraishi et al. [20] (2013) | Mets (RCC) | 2005 to 2011 | PVA, NBCA glue | 25 (68.0) | 59.6 | 90.0 | 68.0 | 0 |
| Kumar et al. [14] (2016) | Mets | 2005 to 2014 | PVA, gelatin sponge | 45 (71.1) | ND | 80.0 | 53.3 | 0 |
| Tang et al. [23] (2018) | Mets, PBT | 2006 to 2012 | Gelatin sponge, coil | 110 (52.7) | 49.6 | 90.0 | 92.8 | 4 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download