Abstract
Objective
This study aims to investigate the incidence of vestibular schwannoma (VS) and demographic characteristics in Korea using population-based National Health Insurance Service data.
Methods
This study analyzed Korean National Health Insurance Service data from 2005 to 2020, based on the International Classification of Diseases, 10th version, Clinical Modification codes D333 and D431. Only those patients who had undergone magnetic resonance imaging and audiologic tests were considered definitive cases. Demographic variables included age, sex, treatment modality, hypertension, diabetics, dyslipidemia, smoking history, alcohol history, and income status.
Results
The total number of VS patients was 5751. The average incidence rate was 0.71 per 100000 from 2005 to 2020, and the annual incidence rate increased from 0.33 in 2005 to 1.32 in 2019 but decreased to 0.80 in 2020. Incidence was highest in those aged 60–69 years (1.791) and lowest in those younger than 20 years (0.041). Incidence was higher in females, and the number of patients who received radiosurgery (46.64%) was largest compared to the wait and scan group (37.96%), microsurgery group (12.85%), or the group who received both (2.56%). Diabetes, dyslipidemia, and alcohol consumption increased the risk of VS, while cigarette smoking reduced the risk of VS.
Vestibular schwannomas (VS), also known as acoustic neuromas, originate from the vestibular branch of the eighth cranial nerve, which is located in the cerebellopontine angle, the space between the brainstem, cerebellum, and temporal bone. Similar to the characteristics of schwannomas, VSs are benign, well-encapsulated, slow-growing nerve sheath tumors that are composed exclusively of Schwann cells [1,2]. VS may lead to asymmetric hearing loss, unilateral tinnitus, and vertigo. A VS can expand out of the internal auditory canal and press on the adjacent nerves, resulting in severe neurological symptoms such as facial palsy and brainstem compression.
Little information exists on the incidence of VS worldwide, but several studies have reported the national incidence rate of VS ranging from approximately 0.6 to 3.3 per 100000 populations [1,2,14,15,24]. Denmark may possess the most complete data set since all VS patients are referred to a single clinic [31]. Since 1976, all patients in Denmark diagnosed with a cerebellopontine angle tumor resembling a VS have been referred to Rigshospitalet (previously Gentofte Hospital), and the patient data were entered prospectively into Denmark’s national database. The estimated incidence increased from 0.26 VS cases per 100000 per year in 1976 to 3.07 VS cases per 100000 per year in 2011. As in Denmark, the incidence rate tends to increase every year in most countries. In four Nordic countries, an overall annual increase of 3% in the incidence rate was observed between 1987 and 2007 [17].
Similar to most other countries, population-based studies of VS have not been reported in Korea. The aim of this study is to investigate the incidence of VS, the associated demographic characteristics in Korea, and the risk factors for VS using population-based National Health Insurance Service data.
This study was approved by the Institutional Review Board of the Catholic university of Korea, Eunpyeong St. Mary's Hospital (No. PC21ZASI0049).
The data of this study are from the Korean National Health Insurance Service from 2005 to 2020. Patients with vestibular schwannoma were identified according to the corresponding International Classification of Diseases, 10th version, Clinical Modification (ICD-10-CM) diagnostic code D333 (benign neoplasm of the brain and other parts of the central nervous system or cranial nerves) or D431 (neoplasm of uncertain origin or unknown behavior of the brain and central nervous system, brain, infratentorial). Inclusion criteria for this study were as follows : 1) diagnostic code D333 or D431; 2) without D32 (benign neoplasm of cerebral meninges), C70 (cerebral meningeal cancer), and C71 (brain cancer); 3) underwent brain magnetic resonance imaging (MRI) or temporal bone MRI within 1 year of diagnosis; and 4) underwent at least one audiologic test (pure tone audiometry, speech audiometry, or auditory brainstem response).
The control group was established using a propensity-score matching method with a fourfold number of the patient group. Annual incidence in total and according to age, sex, and treatment modality (wait and scan, radiosurgery, microsurgery, or both) were analyzed. Chronic metabolic diseases such as hypertension, diabetes, dyslipidemia, smoking history, and alcohol history were investigated to verify whether they were risk factors for VS.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). T-test was used for continuous variables and a chi-square test was used for categorical variables. The risk factors were analyzed by univariate and multivariate Cox proportional hazard regression analyses, where p-value <0.05 was considered to be statistically significant.
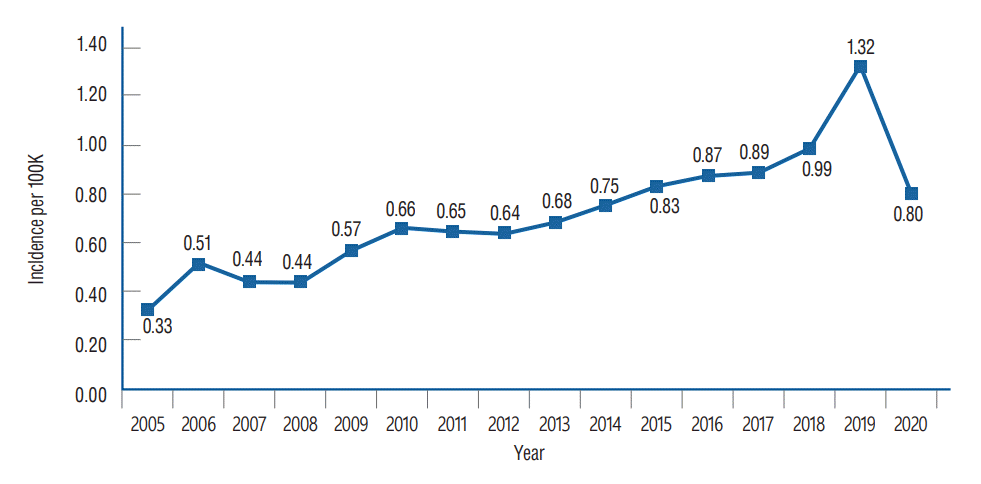
Overall, from 2005 to 2020, there were 5751 patients with VS. Annual incidence rates are shown in Table 1 and Fig. 1. The annual incidence rate had an increasing trend from 0.33 per 100000 in 2005 to 0.80 per 100000 in 2020, with a peak annual incidence of 1.32 per 100000 in 2019. The overall annual incidence rate was 0.71 per 100000.
The characteristic variables of the VS group (n=5751) and the control group (n=23004) are shown in Table 2. The VS group included 2456 male patients (42.7%) and 3295 female patients (57.3%). Age group was divided by 10 years, from below 20 years-old from above 80 years-old. The patients in the 50–59 years age group accounted for the largest portion. The mean age of VS group was 53.42 and the mean age of control group was 53.35.
The annual incidence of VS according to sex and age is shown in Table 3 and Fig. 2. The annual incidence in females was higher than in males for all ages except those 80 years of age or older (female/male ratio, 0.616). The overall female to male incidence ratio was 1.225, and the sex difference in VS was statistically significant (p=0.0098).
The incidence of the total population, males, and females was highest in the 60–69 years age group (1.791, 1.510, and 2.050 per 100000, respectively) and all lowest in those younger than 20 years (0.041, 0.038, and 0.044 per 100000, respectively).
For treatment modalities, patients who received radiosurgery (46.64%) were the most common, followed by the wait and scan group (37.96%), microsurgery group (12.85%), and those who received both (2.56%). Household income was divided into four groups. The fourth quartile (the highest income level) accounted for 41.64% of the VS patients, followed by the third quartile for 22.85% (Table 2).
The chronic metabolic diseases (hypertension, diabetes, and dyslipidemia), smoking history, and alcohol history of VS group and control group is shown in Table 2. The univariate and multivariate Cox proportional hazard regression analysis of risk factors are shown in Table 4. In univariate analysis, VS was associated with all of three metabolic diseases; hypertension (hazard ratio [HR], 1.223; 95% confidence interval [CI], 1.161–1.289), diabetes mellitus (HR, 1.346; 95% CI, 1.274–1.421), dyslipidemia (HR, 1.459; 95% CI, 1.385–1.537). Smoking history decreased the risk of VS (HR, 0.806; 95% CI, 0.755–0.860), and alcohol history increased the risk of VS (HR, 2.069; 95% CI, 1.952–2.194).
However, in multivariate Cox regression analysis, hypertension was not a statistically significant risk factor of VS (p=0.3104). VS was associated with diabetes mellitus (HR, 1.123; 95% CI, 1.048–1.203) and dyslipidemia (HR, 1.276; 95% CI, 1.194–1.363) (Table 4). Alcohol consumption increased the risk of VS (HR, 2.631; 95% CI, 2.469–2.803) in highest HR among the risk factors. On the contrary, smoking (current or ex-smoker) was associated with decreased risk of VS (HR, 0.557; 95% CI, 0.519–0.597).
This study is an epidemiological study of all Korean patients diagnosed with VS using population-based data for the total population of Korea. The overall annual incidence from 2005 to 2020 was 0.71 per 100000, ranging from 0.33 to 1.32. This appeared to be lower than the incidences reported previously in Taiwan (incidence range from 1.74 to 3.72 per 100000 from 2001 to 2012), Netherlands (incidence range from 1.03 to 1.55 per 100000 from 2001 to 2012), and Denmark (incidence range from 0.26 to 3.7 per 100000 from 1976 to 2011) [14,15,17]. Compared to the United States with its overall incidence of 1.14 per 100000 from 2004 to 2016 [8], the overall incidence was lower in the present study.
Several studies have reported that the high incidence rate of VS was due to high medical utilization, high number of outpatient visits, or easy accessibility to specialists. However, in Korea, there is a greater possibility of utilization of the health care system with higher rates of consultation to specialists. According to the Organisation for Economic Co-operation and Development (OECD) report, Korea had 16.6 hospital visits per population in 2017, which is the highest among OECD countries. The average number of doctor consultations per person for other member countries was 7.1 in 2017 [21]. Remote areas that have limited access to medical institutions are rare, and MRI examinations are covered by national health insurance. Recently, the knowledge of VS pathology has significantly increased due to inactivation of the NF2 gene and its product merlin. Different genetic backgrounds and ethnicities may account for the variation in incidence rates [6,10] but are unlikely to directly explain the low incidence rates in our study. Strict inclusion criteria of our study are believed to be related with the low incidence. Patients who did not receive audiologic test evaluations were omitted from our study.
Although the incidence rate varies by country, they are steadily increasing worldwide. In our study, the annual incidence rate gradually increased from 0.33/100000 in 2005 to 1.32/100000 in 2019. A possible main reason for this increase is improved access to sensitive diagnostic tools, especially MRI. The size of tumors at diagnosis remarkably decreased from 30 mm in the mid-1970s to 10 mm in the 2000s [8]. Increased awareness toward hearing impairment among patients is likely to be a contributing factor, leading to frequent audiologic screening tests and more accidental findings of VS. The finding that the upper 50% of the income group accounts for 63% of VS patients (Table 1) supports this opinion. However, increases in tumor occurrence itself cannot be completely ruled out [19].
In 2018, the Korean national health insurance coverage for temporal bone MRI was expanded to unilateral hearing loss worse than 40 dB or tinnitus lasting more than 6 months. Therefore, decreases in cost burden may contribute to the high increase of VS patients in 2019. In 2020, the annual incidence decreased to 0.80/100000 compared to 1.32/100000 in 2019. This sudden decrease may be due to the effects of the coronavirus disease 2019 (COVID-19). Many other diseases such as breast cancer also exhibited a decline of diagnosis in 2020, which demonstrated a significant statistical difference compared to 2019 [12]. To our knowledge, ours is the first VS study including data from the year 2020. The COVID-19 pandemic may have significantly impacted the rates of screening and case identification and will likely show a similar decline in other countries in 2020.
The annual incidence of VS was highest in the age range of 60 to 69 years (1.791/100000 persons) and second highest in the age range of 50 to 59 years (1.497/100000 persons). This result is in line with other countries where the incidence rates peaked in the sixth [15] or seventh decade of age [1]. The annual incidence of VS in female patients (0.900) was higher than in male patients (0.734) as seen in Table 3. The overall female to male incidence ratio was 1.225, and the sex difference was statistically significant (p=0.0098). A study in Taiwan reported the female to male incidence ratio as 1.314 [15]. Compared to Asian countries, Western Europe and North America reported small sex differences. Carlson et al. [6] reported a female proportion of 52.1% (year of 1973 to 2012) and Cioffi et al. [8] reported it as 52.6% (year of 2004 to 2016). Kleijwegt et al. [14] reported Netherland’s female proportion of 50.2%. Denmark was the only country that reported a higher male proportion (50.3%), but the difference was not statistically significant [25].
There are currently three main approaches for diagnosed VS patient. In our study, patients who received radiosurgery (46.64%) were the majority, followed by the wait and scan group (37.96%), microsurgery group (12.85%), and both (2.56%). Stereotactic radiosurgery (SRS) has become an important treatment modality since its introduction in 1969, because it is less invasive and has been associated with a good tumor control rate and low morbidity [13]. The common indications for SRS are a lesion with a diameter less than 3 cm, a small lesion limited to the internal acoustic canal, or a recurred lesion after microsurgery [18]. Nowadays, the tumor size at the time of diagnosis has decreased over time due to easy access to MRI and hearing screening tests [5]. This early diagnosis may have contribute to the shift of treatment trend to SRS and observation. In addition older patients with VS are more likely to undergo radiosurgery in aging society. In the other study using United States National Cancer Database from 2004 to 2011, 48% received primary microsurgery (13.4% with postoperative radiation therapy), 24% received radiation therapy alone, and 29% wait and scan [5]. Compared to this United States database from 2004 to 2011, Korea database from 2005 to 2020 had the treatment trend focused on radiosurgery and observation.
The etiology of VS remains largely unknown. In our study, diabetes mellitus, dyslipidemia, and alcohol consumption were associated with a higher risk of VS but exhibited no significant association with hypertension (Table 4). Hypertension, diabetes mellitus, and dyslipidemia as VS risk factors have been rarely studied. In studies of brain tumors, several have reported that hypertension and diabetes mellitus were positively associated with the incidence of brain tumors [27,30], while others reported a null [4,26] or inverse association [3]. In an earlier study, VS risk was not related to consumption of alcohol [9]. Other possible predisposing factors for VS such as ionized radiation, radio frequency electromagnetic fields, noise exposure, and allergic diseases were observed [7,23,28].
On the contrary, ever-smokers (current or ex-smoker) showed a low risk of VS compared to non-smokers in this study. Cigarette smoking has not generally been associated with reduction of risk of central nervous system or brain tumors [11]. However, several studies have reported that smoking decreased the risk of VS [2,7]. An international case-control study by Schoemaker et al. [29] in the United Kingdom and Nordic countries also reported that current smokers and those who had recently stopped smoking showed reduced risk of VS. There are some possible mechanisms of cigarettes for the low risk of VS. Smoking has hypoxic effects on tissue, and an in vitro study demonstrated a significant decrease of Schwann cell survival under hypoxic conditions, reducing brain derived neurotrophic factor mRNA expression that is significantly up-regulated in VS [16,33]. In addition, nicotine is responsible for anti-inflammatory effects, suppressing the severity of experimental autoimmune encephalomyelitis in both in vitro and in vivo animal studies [20,22].
Several limitations of this study should be considered. First, misclassification of cases of VS is possible based on the patient’s diagnostic codes, resulting in the exclusion of patients with VS. In addition, VS patients may have been erroneously assigned a different diagnostic code such as other brain tumors instead of D333 or D431. Patients with other misdiagnosed tumors of the brain or cranial nerves could be erroneously included through miscoding of D333 or D431. Second, since only patients who had received audiologic tests and MRIs within 1 year from the time of diagnosis were considered as definitive cases to improve accuracy, VS patients without audiologic tests or MRI within 1 year were omitted from our data. Third, although the National Health Insurance Service database contains abundant claims data of Korea, it does not provide accurate data for smoking and alcohol consumption. In addition, smoking status, amount of smoking, type of cigarettes, and time of smoking exposure are not collected. Therefore, among the risk variables of this study, smoking and alcohol should be interpreted with missing value bias considerations [32]. Nonetheless, this study analyzed population-based data including the entire population in Korea and investigated the incidence and risk factors for VS. This most recent epidemiological study of VS could be helpful for clinicians to investigate and treat the disease.
Notes
ACKNOWLEDGMENTS
This study was supported by the Research Fund of the E.N.T. Catholic University of Korea that was created in the program year of 2020.
References
1. Babu R, Sharma R, Bagley JH, Hatef J, Friedman AH, Adamson C. Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management. J Neurosurg. 119:121–130. 2013.

2. Berkowitz O, Iyer AK, Kano H, Talbott EO, Lunsford LD. Epidemiology and environmental risk factors associated with vestibular schwannoma. World Neurosurg. 84:1674–1680. 2015.

3. Bernardo BM, Orellana RC, Weisband YL, Hammar N, Walldius G, Malmstrom H, et al. Association between prediagnostic glucose, triglycerides, cholesterol and meningioma, and reverse causality. Br J Cancer. 115:108–114. 2016.

4. Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 99:252–259. 2002.

5. Carlson ML, Habermann EB, Wagie AE, Driscoll CL, Van Gompel JJ, Jacob JT, et al. The changing landscape of vestibular schwannoma management in the United States--a shift toward conservatism. Otolaryngol Head Neck Surg. 153:440–446. 2015.

6. Carlson ML, Marston AP, Glasgow AE, Habermann EB, Sweeney AD, Link MJ, et al. Racial differences in vestibular schwannoma. Laryngoscope. 126:2128–2133. 2016.

7. Chen M, Fan Z, Zheng X, Cao F, Wang L. Risk factors of acoustic neuroma: systematic review and meta-analysis. Yonsei Med J. 57:776–783. 2016.

8. Cioffi G, Yeboa DN, Kelly M, Patil N, Manzoor N, Greppin K, et al. Epidemiology of vestibular schwannoma in the United States, 2004-2016. Neurooncol Adv. 2:vdaa135. 2020.

9. Corona AP, Ferrite S, Lopes Mda S, Rêgo MA. Risk factors associated with vestibular nerve schwannomas. Otol Neurotol. 33:459–465. 2012.

10. de Vries M, van der Mey AG, Hogendoorn PC. Tumor biology of vestibular schwannoma: a review of experimental data on the determinants of tumor genesis and growth characteristics. Otol Neurotol. 36:1128–1136. 2015.
11. Efird JT, Friedman GD, Sidney S, Klatsky A, Habel LA, Udaltsova NV, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 68:57–69. 2004.

12. Kang YJ, Baek JM, Kim YS, Jeon YW, Yoo TK, Rhu J, et al. Impact of the COVID-19 pandemic on the diagnosis and surgery of breast cancer: a multi-institutional study. J Breast Cancer. 24:491–503. 2021.

13. Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG. Long-term outcomes of gamma knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc. 42:286–292. 2007.

14. Kleijwegt M, Ho V, Visser O, Godefroy W, van der Mey A. Real incidence of vestibular schwannoma? Estimations from a national registry. Otol Neurotol. 37:1411–1417. 2016.

15. Koo M, Lai JT, Yang EY, Liu TC, Hwang JH. Incidence of vestibular schwannoma in Taiwan from 2001 to 2012: a population-based national health insurance study. Ann Otol Rhinol Laryngol. 127:694–697. 2018.

16. Kramer F, Stöver T, Warnecke A, Diensthuber M, Lenarz T, Wissel K. BDNF mRNA expression is significantly upregulated in vestibular schwannomas and correlates with proliferative activity. J Neurooncol. 98:31–39. 2010.

17. Larjavaara S, Feychting M, Sankila R, Johansen C, Klaeboe L, Schüz J, et al. Incidence trends of vestibular schwannomas in Denmark, Finland, Norway and Sweden in 1987-2007. Br J Cancer. 105:1069–1075. 2011.

18. Lim YJ, Choi SK. Gamma knife radiosurgery for vestibular schwannomas. J Korean Neurosurg Soc. 42:159–167. 2007.
19. Marinelli JP, Lohse CM, Grossardt BR, Lane JI, Carlson ML. Rising incidence of sporadic vestibular schwannoma: true biological shift versus simply greater detection. Otol Neurotol. 41:813–847. 2020.

20. Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 183:6681–6688. 2009.

21. OECD. OECD reviews of public health: Korea: a healthier tomorrow, OECD reviews of public health. Paris: OECD Publishing;2020.
22. Palmisano S, Schwartzbaum J, Prochazka M, Pettersson D, Bergenheim T, Florentzson R, et al. Role of tobacco use in the etiology of acoustic neuroma. Am J Epidemiol. 175:1243–1251. 2012.

23. Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 94:1555–1563. 2002.

24. Propp JM, McCarthy BJ, Davis FG, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro Oncol. 8:1–11. 2006.
25. Reznitsky M, Petersen MMBS, West N, Stangerup SE, Cayé-Thomasen P. Epidemiology and diagnostic characteristics of vestibular schwannomas-does gender matter? Otol Neurotol. 41:e1372–e1378. 2020.

26. Schlehofer B, Blettner M, Preston-Martin S, Niehoff D, Wahrendorf J, Arslan A, et al. Role of medical history in brain tumour development. results from the international adult brain tumour study. Int J Cancer. 82:155–160. 1999.

27. Schneider B, Pülhorn H, Röhrig B, Rainov NG. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detect Prev. 29:440–447. 2005.

28. Schoemaker MJ, Swerdlow AJ, Ahlbom A, Auvinen A, Blaasaas KG, Cardis E, et al. Mobile phone use and risk of acoustic neuroma: results of the interphone case-control study in five North European countries. Br J Cancer. 93:842–848. 2005.
29. Schoemaker MJ, Swerdlow AJ, Auvinen A, Christensen HC, Feychting M, Johansen C, et al. Medical history, cigarette smoking and risk of acoustic neuroma: an international case-control study. Int J Cancer. 120:103–110. 2007.
30. Seliger C, Meier CR, Becker C, Jick SS, Proescholdt M, Bogdahn U, et al. Metabolic syndrome in relation to risk of meningioma. Oncotarget. 8:2284–2292. 2017.
31. Stepanidis K, Kessel M, Caye-Thomasen P, Stangerup SE. Sociodemographic distribution of vestibular schwannomas in Denmark. Acta Otolaryngol. 134:551–556. 2014.
32. Zhang Z. Missing values in big data research: some basic skills. Ann Transl Med. 3:323. 2015.
Fig. 2.
Age- and sex-specific annual incidence of vestibular schwannoma per 100000 persons according to the Korean National Health Insurance Service Data, 2005 to 2020.
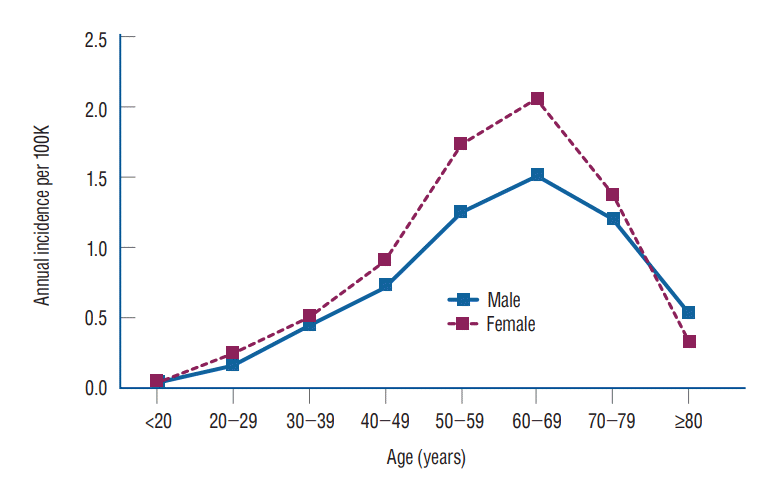
Table 1.
Incidence of vestibular schwannoma according to Korean National Health Insurance service data, 2005 to 2020
| Year | Incident cases | Population* | Annual incidence per 100000 (95% CI) |
|---|---|---|---|
| 2005 | 159 | 48683039.5 | 0.33 (0.28–0.39) |
| 2006 | 251 | 48887026.5 | 0.51 (0.45–0.58) |
| 2007 | 216 | 49130353.5 | 0.44 (0.39–0.50) |
| 2008 | 217 | 49404647.5 | 0.44 (0.39–0.50) |
| 2009 | 281 | 49656756.0 | 0.57 (0.50–0.63) |
| 2010 | 329 | 49879811.5 | 0.66 (0.59–0.73) |
| 2011 | 324 | 50111475.5 | 0.65 (0.58–0.72) |
| 2012 | 322 | 50345324.5 | 0.64 (0.57–0.71) |
| 2013 | 346 | 50558951.5 | 0.68 (0.61–0.76) |
| 2014 | 382 | 50763158.0 | 0.75 (0.68–0.83) |
| 2015 | 424 | 50951719.0 | 0.83 (0.75–0.91) |
| 2016 | 447 | 51112971.5 | 0.87 (0.79–0.96) |
| 2017 | 456 | 51230704.0 | 0.89 (0.81–0.97) |
| 2018 | 506 | 51300879.5 | 0.99 (0.90–1.07) |
| 2019 | 679 | 51337423.5 | 1.32 (1.22–1.42) |
| 2020 | 412 | 51349259.0 | 0.80 (0.72–0.88) |
| Total | 5751 | 804703500.5 | 11.38 |
| Mean | 359.44 | 50293968.8 | 0.71 |
Table 2.
Characteristics of the vestibular schwannoma group and the control group
Table 3.
Age- and sex-specific annual incidence of vestibular schwannoma per 100000 according to the Korean National Health Insurance Service Data, 2005 to 2020
Table 4.
Risk factors of vestibular schwannoma (results of Cox-regression analysis)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download