INTRODUCTION
METHODS
Patients and baseline procedures
Weight changes assessment
Follow-up and outcome assessment
Statistical analyses
RESULTS
Baseline characteristics according to relative body weight changes
Table 1.
| Characteristic | All patients (n=651) | Weight loss ≥5% (n=125) | Weight loss 0% to <5% (n=180) | Weight gain (n=346) | P value | |
|---|---|---|---|---|---|---|
| Age, yr | 59.9±9.5 | 60.0±10.1 | 59.9±9.8 | 59.9±9.2 | 0.990 | |
| Male sex, % | 38.1 | 31.2 | 39.4 | 39.9 | 0.210 | |
| Body mass index, kg/m2 | 29.7±4.9 | 30.9±4.8a | 29.9±5.1 | 29.1±4.7 | 0.002 | |
| Abdominal circumference, cm | 102±11 | 105±11a | 102±12 | 101±11 | 0.003 | |
| Smoking, current/past, % | 43.8 | 40.0 | 39.4 | 47.4 | 0.141 | |
| Physical activity, % active | 34.4 | 35.2 | 31.8 | 35.4 | 0.710 | |
| Diabetes duration, yr | 8 (3–15) | 5 (2–12)b | 7 (3–12) | 10 (4–18) | <0.001 | |
| Chronic diabetic complication, % | ||||||
| Cerebrovascular disease | 8.4 | 8.8 | 8.3 | 8.4 | 0.990 | |
| Coronary artery disease | 14.9 | 11.2 | 15.6 | 15.9 | 0.432 | |
| Peripheral artery disease | 16.3 | 12.0 | 16.8 | 17.6 | 0.341 | |
| Retinopathy | 31.9 | 26.4 | 27.0 | 36.3 | 0.038 | |
| Nephropathy | 30.8 | 23.8 | 31.4 | 32.9 | 0.170 | |
| Peripheral neuropathy | 28.1 | 21.1 | 27.0 | 31.3 | 0.092 | |
| Diabetes treatment, % | ||||||
| Metformin | 88.0 | 84.8 | 88.3 | 89.0 | 0.461 | |
| Sulfonylureas | 42.5 | 46.4 | 50.6a | 37.0 | 0.007 | |
| Insulin | 47.5 | 32.8b | 38.9b | 57.2 | <0.001 | |
| Other medicationsc | 5.3 | 5.7 | 5.1 | 5.2 | 0.971 | |
| Dyslipidemia, % | 87.1 | 82.4 | 88.3 | 88.2 | 0.220 | |
| Statin use | 77.1 | 70.4 | 78.3 | 78.9 | 0.141 | |
| Arterial hypertension, % | 86.3 | 87.2 | 85.6 | 86.4 | 0.921 | |
| Number of antihypertensive drugs | 3 (1–3) | 2 (1–3) | 3 (1–4) | 3 (1–3) | 0.601 | |
| ACE inhibitors/AR blockers, % | 81.0 | 80.8 | 78.3 | 82.4 | 0.531 | |
| Diuretics, % | 61.4 | 62.4 | 60.6 | 61.6 | 0.951 | |
| Calcium channel blockers, % | 28.1 | 25.6 | 29.4 | 28.3 | 0.760 | |
| Beta-blockers, % | 45.5 | 42.4 | 48.3 | 45.1 | 0.580 | |
| Mean 2-year SBP, mm Hg | 140±18 | 139±19 | 141±18 | 141±17 | 0.711 | |
| Mean 2-year DBP, mm Hg | 79±9 | 79±9 | 79±9 | 79±9 | 0.990 | |
| Laboratory variables | ||||||
| Mean fasting glycemia, mmol/L | 8.2±2.8 | 7.6±2.8 | 8.4±2.9 | 8.2±2.8 | 0.028 | |
| Mean HbA1c, % | 7.7±1.4 | 7.2±1.4b | 7.7±1.4 | 7.9±1.4 | <0.001 | |
| Mean HbA1c, mmol/mol | 61±15.3 | 55±15.3 | 61±15.3 | 63±15.3 | ||
| Mean triacylglycerol, mmol/L | 1.9±1.4 | 1.6±0.9a | 2.0±1.3 | 1.9±1.6 | 0.004 | |
| Mean HDL-C, mmol/L | 1.1±0.3 | 1.1±0.3 | 1.2±0.3 | 1.1±0.2 | 0.941 | |
| Mean LDL-C, mmol/L | 2.7±0.8 | 2.7±0.9 | 2.7±0.7 | 2.7±0.8 | 0.920 | |
| eGFR, mL/min/1.73 m2 | 80±18 | 81±17 | 82±18 | 79±17 | 0.151 | |
| Albuminuria, mg/day | 13 (7–40) | 11 (7–24) | 15 (7–40) | 13 (7–50) | 0.350 | |
Values are expressed as mean±standard deviation, percentage, or median (interquartile range).
ACE, angiotensin-converting enzyme; AR, angiotensin II receptor; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate.
Post hoc comparisons among subgroups were performed against the reference subgroup (weight gain) with the Bonferroni correction for multiple comparisons
Follow-up and incidence of adverse outcomes
Fig. 1.
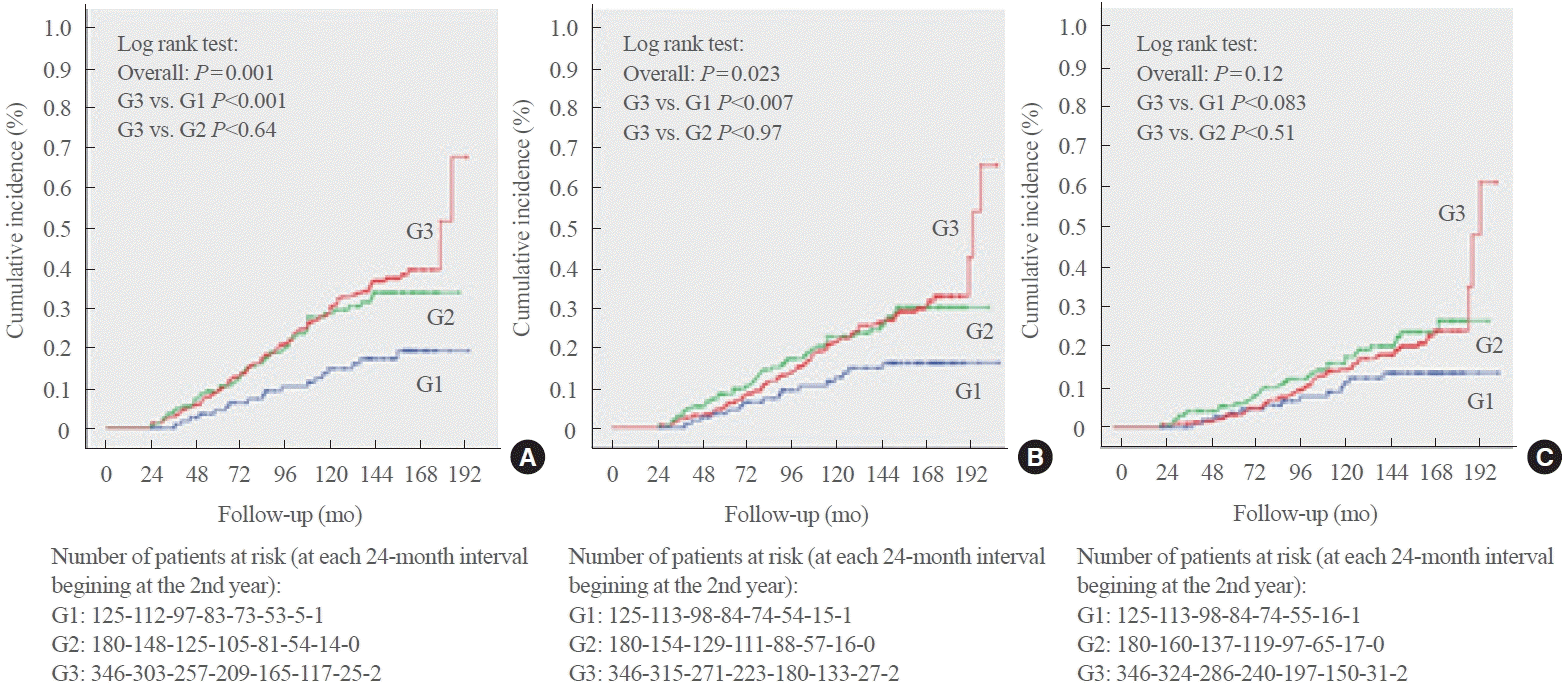
Table 2.
| Outcomes incidence | All patients (n=651) | Weight loss ≥5% (n=125) | Weight loss 0% to <5% (n=180) | Weight gain (n=346) | P value |
|---|---|---|---|---|---|
| Total cardiovascular events | 188 (31.6) | 19 (15.3)a | 53 (33.7) | 116 (37.0) | 0.001 |
| Major adverse cardiovascular events | 150 (24.3) | 17 (13.6)b | 43 (26.3) | 90 (27.4) | 0.023 |
| Cardiovascular mortality | 106 (16.5) | 13 (10.3) | 33 (19.2) | 60 (17.4) | 0.121 |
Adjusted risks associated with changes in body weight
Table 3.
Model 1 was adjusted for age, sex, baseline body mass index, smoking status, diabetes duration, presence of macrovascular and microvascular complications, use of insulin and statins, and number of antihypertensive drugs. Model 2 was further adjusted for the potential mediating covariates between weight loss during the initial 2-year period and outcomes: regular physical activity, and mean systolic blood pressure, glycated hemoglobin, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.
HR, hazard ratio; CI, confidence interval.
Table 4.
HRs were adjusted for age, sex, baseline body mass index, smoking status, diabetes duration, presence of macrovascular and microvascular complications, use of insulin and statins, number of antihypertensive drugs, and mean systolic blood pressure, glycated hemoglobin, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol during the 2-year period of weight change (model 2 in Table 2).
HR, hazard ratio; CI, confidence interval.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download