INTRODUCTION
METHODS
Data sources and searches
Inclusion and exclusion criteria
Quality assessment
Data extraction and data conversion
Table 1.
| Study | Sex | Age (mean) | Continent (country) | Group (n; SAA level mean±SD/ median value [IQR]) | BMI in T2DM patients (mean±SD), kg/m2 | Correlation of SAA with other factors in T2DM patients | Study type | Method | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Ebtehaj et al. (2017) [22] | 27 Men and 49 women | 43–70 (59) | Europe (Netherlands) | Case (40; 1.71 [1.25–2.48] mg/L) | 28.9±4.9 | NA | Cross-sectional | ELISA | ****** |
| Control (36; 1.58 [0.82–2.18] mg/L) | |||||||||
| Yang et al. (2017) [23] | 125 Men and 261 women | 44–63 (54) | Asia (China) | Case (185; 892 [537–1,129] μg/L) | 26.8±4.0 | NA | Case-control | NA | ****** |
| Control (201; 657 [462–831] μg/L) | |||||||||
| Griffiths et al. (2017) [24] | 84 Women | 28–49 (37) | Europe (Ireland) | Case (42; 13 [8–29] μg/L) | 35.88±7.72 | SAA and BMI, FPG, HbA1c, HDL-C (r=0.48, 0.294, 0.348, –0.197) | Case-control | ELISA | ******* |
| Control (42; 6 [3–13] μg/L) | |||||||||
| Zhao et al. (2016) [25] | 299 Men and 299 women | 45–65 (54) | Asia (China) | Case (300; 928.5±326.8 μg/L) | 26.4±3.7 | NA | Case-control | ELISA | ****** |
| Control (298; 811.9±286.8 μg/L) | |||||||||
| Moura Neto et al. (2014) [26] | 52 Men and 88 women | 50–63 (57) | South America (Brazil) | Case (70; 14.68 [5.92–26.04] μg/mL) | 30.0±6.44 | NA | Cross-sectional | ELISA | ******* |
| Control (70; 10.72 [3.67–19.11] μg/mL) | |||||||||
| Leinonen et al. (2004) [31] | 144 Men and 102 women | 52–67 (60) | Europe (Finland) | Case (168; 23 [4.8–2,082] μg/mL) | 30.4±5.4 | NA | Cross-sectional | ELISA | **** |
| Control (78; 19 [5.1–153] μg/mL) | |||||||||
| Morgantini et al. (2011) [32] | 22 Men and 28 women | 52–74 (62) | Europe (Italy) | Case (26; 48.2±35.1 μg/dL) | 34.0±8.0 | NA | Cross-sectional | ELISA | ***** |
| Control (24; 22.7±1.5 μg/dL) | |||||||||
| Murakami et al. (2013) [33] | 25 Men and 20 women | 30–73 (54) | Asia (Japan) | Case (36; 2.69±1.75 μg/mL) | NA | NA | Cross-sectional | ELISA | **** |
| Control (9; 3.22±2.05 μg/mL) | |||||||||
| Yassine et al. (2015) [34] | 76 Men and 84 women | 36–69 (53) | North America (USA) | Case (91; 21.2 [9.9–38.6] ng/mL) | 33.9±8.4 | NA | Cross-sectional | ELISA | ***** |
| Control (69; 28.7 [17.3–44.5] ng/mL) | |||||||||
| Hatanaka et al. (2007) [27] | 17 Men and 21 women | 43–75 (60) | South America (Brazil) | Case (18; 3,443.4±5,036.0 pg/mL) | 26.5±3.5 | NA | Case-control | ELISA | ****** |
| Control (20; 1,179±1,235.2 pg/mL) | |||||||||
| Tsun et al. (2013) [35] | 90 Men and 295 women | 38–59 (47) | Asia (China) | Case (110; 115.6 [66.1–151.1] ng/mL) | 25.2±3.7 | NA | Cross-sectional | ELISA | ***** |
| Control (275; 106.5 [79.4–137.2] ng/mL) | |||||||||
| Du et al. (2008) [36] | 7 Men and 15 women | 60–72 (66) | Asia (China) | Case (10; 3.34±2.32 g/mL) | 26.5±1.83 | NA | Case-control | ELISA | ***** |
| Control (12; 0.95±0.41 g/mL) | |||||||||
| Stettler et al. (2009) [] | 553 Men and 158 women | 53–70 (61) | Europe (Ireland) | Case (159; 3.15 [2.05–4.9] mg/L) | 29.2±4.63 | NA | Cross-sectional | Immunonephelometry | ***** |
| Control (552; 2.65 [1.60–4.60] mg/L) | |||||||||
| Turgutalp et al. (2013) [38] | 74 Men and 72 women | 40–45 (42) | Asia (Turkey) | Case (62; 5.8±1.33 mg/mL) | NA | NA | Cross-sectional | Immunonephelometry | *** |
| Control (84; 3.8±1.33 mg/mL) | |||||||||
| Karlsson et al. (2004) [28] | 21 Men | 56–60 (58) | Europe (Sweden) | Case (10; 3.16±1.71 mg/mL) | 28.0±1.0 | NA | Case-control | Immunonephelometry | ****** |
| Control (11; 2.22±1.03 mg/mL) | |||||||||
| Kumon et al. (1994) [39] | 89 Men and 107 women | 46–74 (59) | Asia (Japan) | Case (105; 2.1±1.3 mg/L) | NA | NA | Case-control | ELISA | **** |
| Control (91; 1.2±0.5 mg/L) | |||||||||
| Wu et al. (2007) [40] | NA | NA | North America (USA) | Case (31; 5.25±7.9 mg/L) | NA | NA | Cross-sectional | ELISA | ***** |
| Control (23; 2.4±2.1 mg/L) | |||||||||
| Chen et al. (2013) [41] | NA | 42–58 (55) | Asia (China) | Case (112; 318.31±34.35 μg/L) | 26.3±3.7 | SAA and BMI, TG, HDL-C, LDL-C, TC, HbA1c, SBP, DBP (r=–0.069, 0.112, –0.120, 0.559, 0.527, 0.198, 0.615, 0.507) | Case-control | ELISA | **** |
| Control (86; 163.90±37.31 μg/L) | |||||||||
| Muller et al. (2002) [29] | 145 Men and 87 women | 60–71 (65) | Europe (Germany) | NA | 29.7±6.65 | SAA and IL-6, CRP (r=0.35, 0.6) | Case-control | Immunonephelometry | ****** |
| Leinonen et al. (2003) [42] | 163 Men and 76 women | 54–67 (61) | Oceania, Europe (Australia, New Zealand and Finland) | NA | 30.51±5.63 | SAA and BMI, WC, HbA1c, HOMA-IR, CRP, IL-6 (r=0.277, 0.347, 0.232, 0.307, 0.687, 0.449) | Cross-sectional | ELISA | ***** |
| Catalan et al. (2007) [30] | 25 Women | 33–46 (38) | Europe (Spain) | NA | 39.77±10.01 | SAA and BMI, FPG, insulin, HOMA-IR, TG, TC, LDL-C, HDL-C (r=0.66, 0.10, 0.40, 0.27, 0.25, –0.08, –0.15, –0.55) | Case-control | ELISA | ****** |
SAA, serum amyloid A; SD, standard deviation; IQR, interquartile range; BMI, body mass index; T2DM, type 2 diabetes mellitus; NA, not applicable; ELISA, enzyme-linked immune sorbent assay; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; IL-6, interleukin-6; CRP, C-reactive protein; WC, waist circumference; HOMA-IR, homeostasis model assessment for insulin resistance.
Statistical methods
RESULTS
Characteristics of included studies
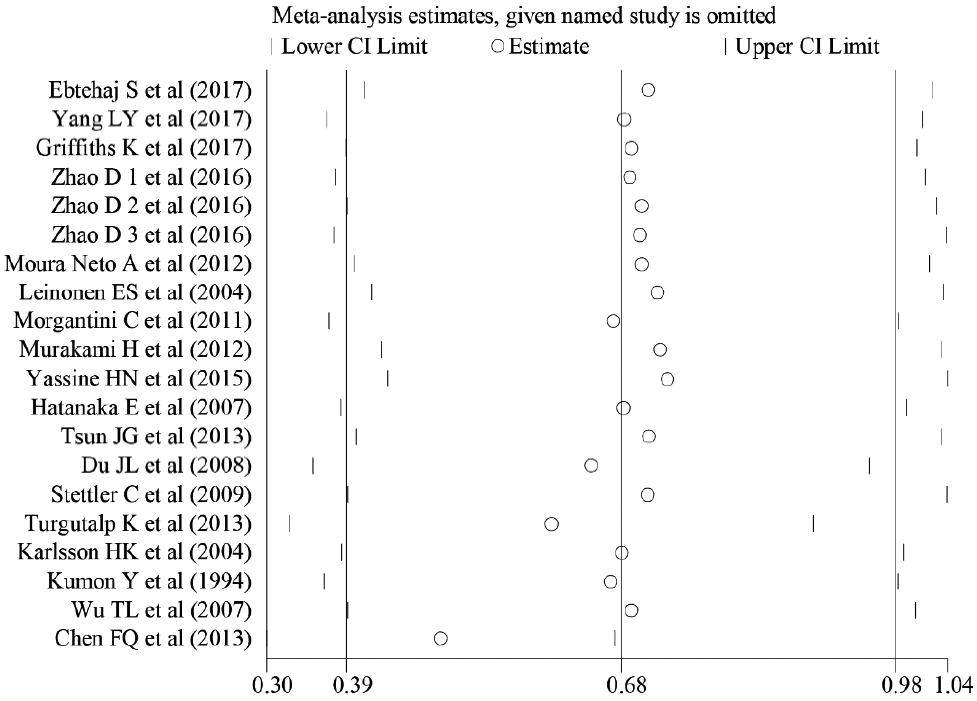
Meta-analyses of SAA and T2DM
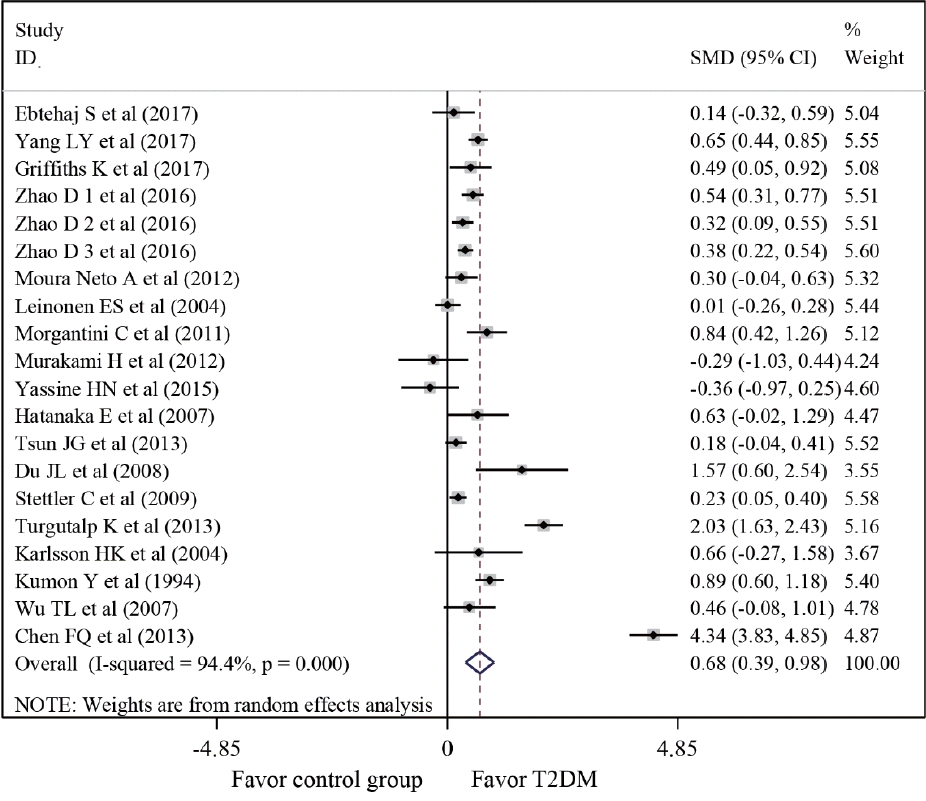 | Fig. 2.Forest plot of differences in serum amyloid A levels between patients with type 2 diabetes mellitus (T2DM) and healthy controls. SMD, standardized mean difference; CI, confidence interval. |
Table 2.
Table 3.
| Covariates | β | SE | T | P>| T | | 95% CI | Tau2 | I2 | Adjusted R2, %a |
|---|---|---|---|---|---|---|---|---|
| Sex | 0.12 | 0.37 | 0.33 | 0.74 | –0.65 to 0.90 | 1.00 | 94.65 | –5.21 |
| Mean age | –0.04 | 0.30 | –0.15 | 0.88 | –0.68 to 0.59 | 1.01 | 94.52 | –5.79 |
| BMI | –0.33 | 0.30 | –1.10 | 0.29 | –0.97 to 0.30 | 0.94 | 93.85 | 1.74 |
| Study design | –0.34 | 0.22 | –0.53 | 0.15 | –0.79 to 0.13 | 0.90 | 94.29 | 6.28 |
| SAA detection method | 0.24 | 0.48 | 0.49 | 0.63 | –0.78 to 1.25 | 1.00 | 94.65 | –4.52 |
| Continent | 0.22 | 0.22 | 0.97 | 0.35 | –0.25 to 0.68 | 0.96 | 94.36 | –0.37 |
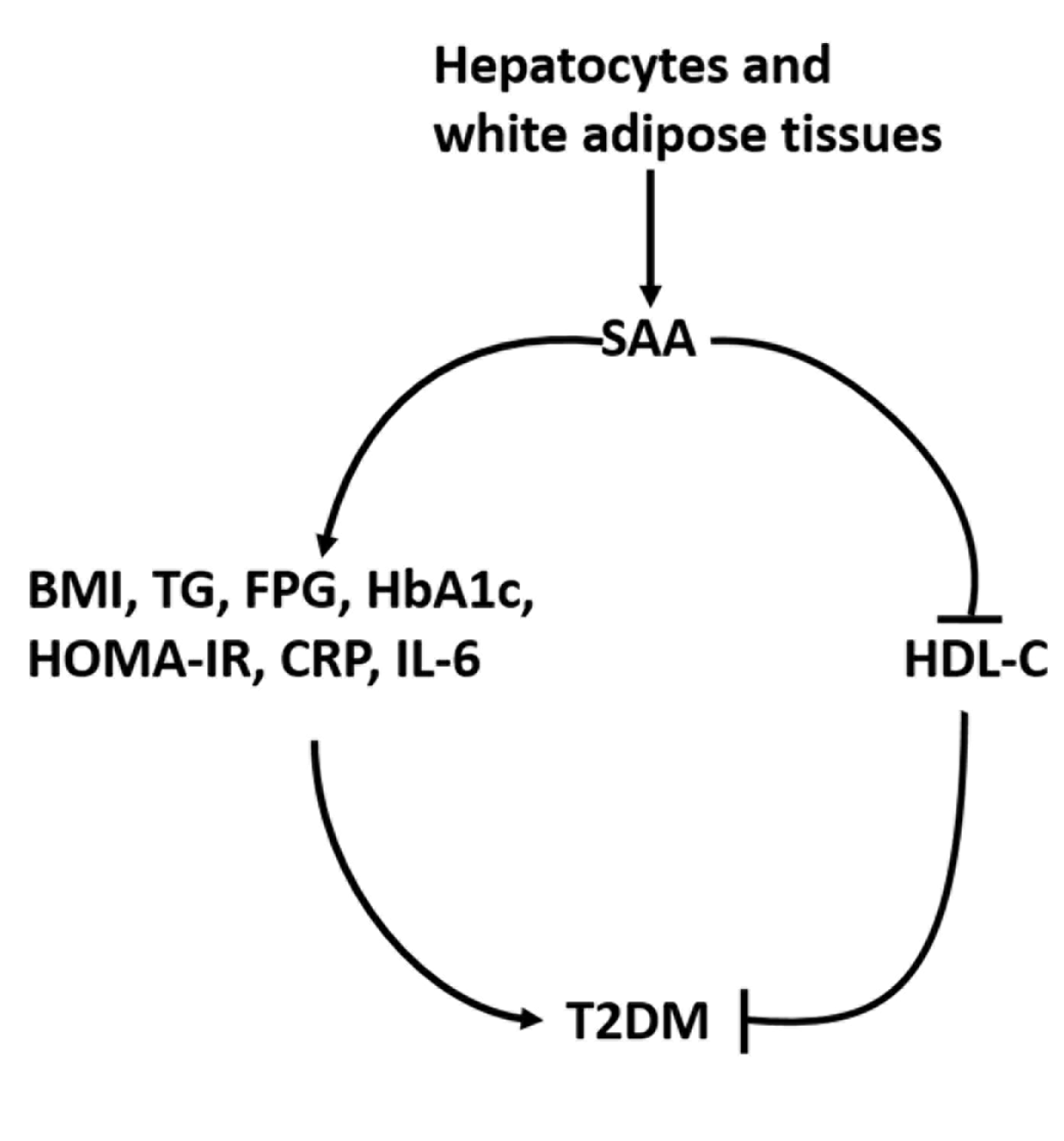
Meta-analysis of correlations between SAA and several factors in T2DM
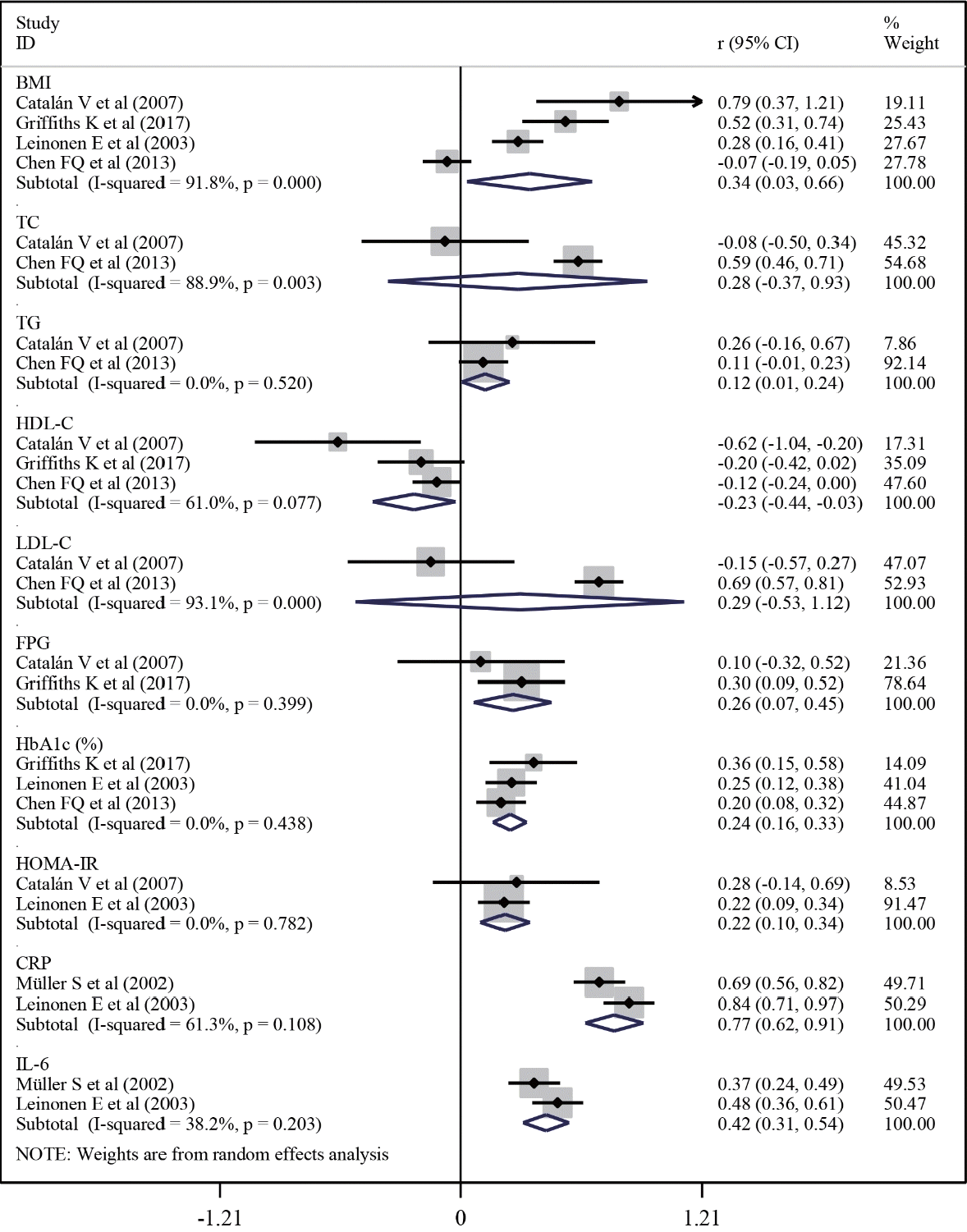 | Fig. 4.Correlations of serum amyloid A levels with cardiometabolic risk factors in patients with type 2 diabetes mellitus. CI, confidence interval; BMI, body mass index; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment for insulin resistance; CRP, C-reactive protein; IL-6, interleukin-6. |
Publication biases
Table 4.
SAA, serum amyloid A; T2DM, type 2 diabetes mellitus; BMI, body mass index; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin A1c; HOMA-IR, homeostasis model assessment for insulin resistance; CRP, C-reactive protein; IL-6, interleukin-6.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download