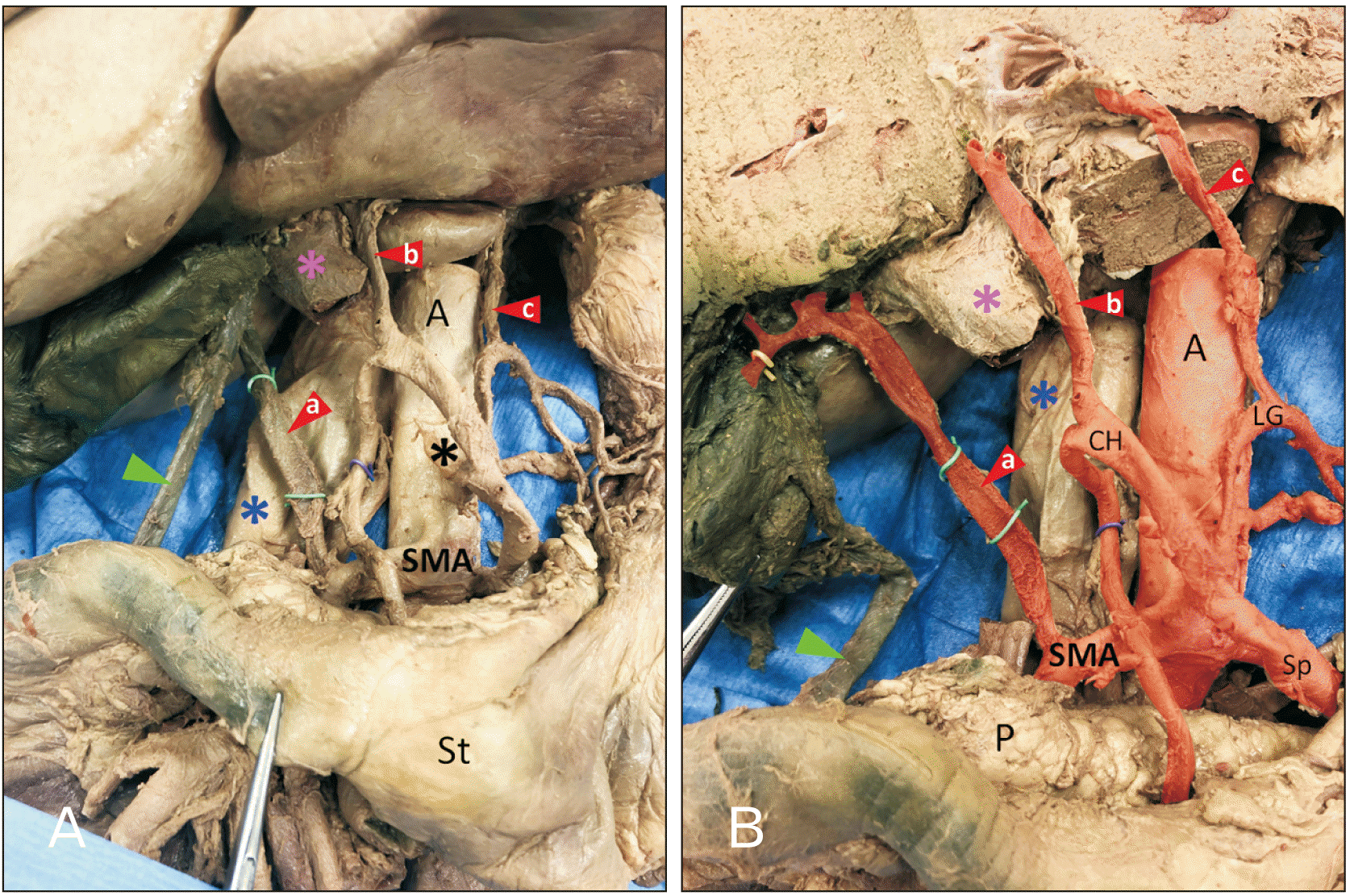
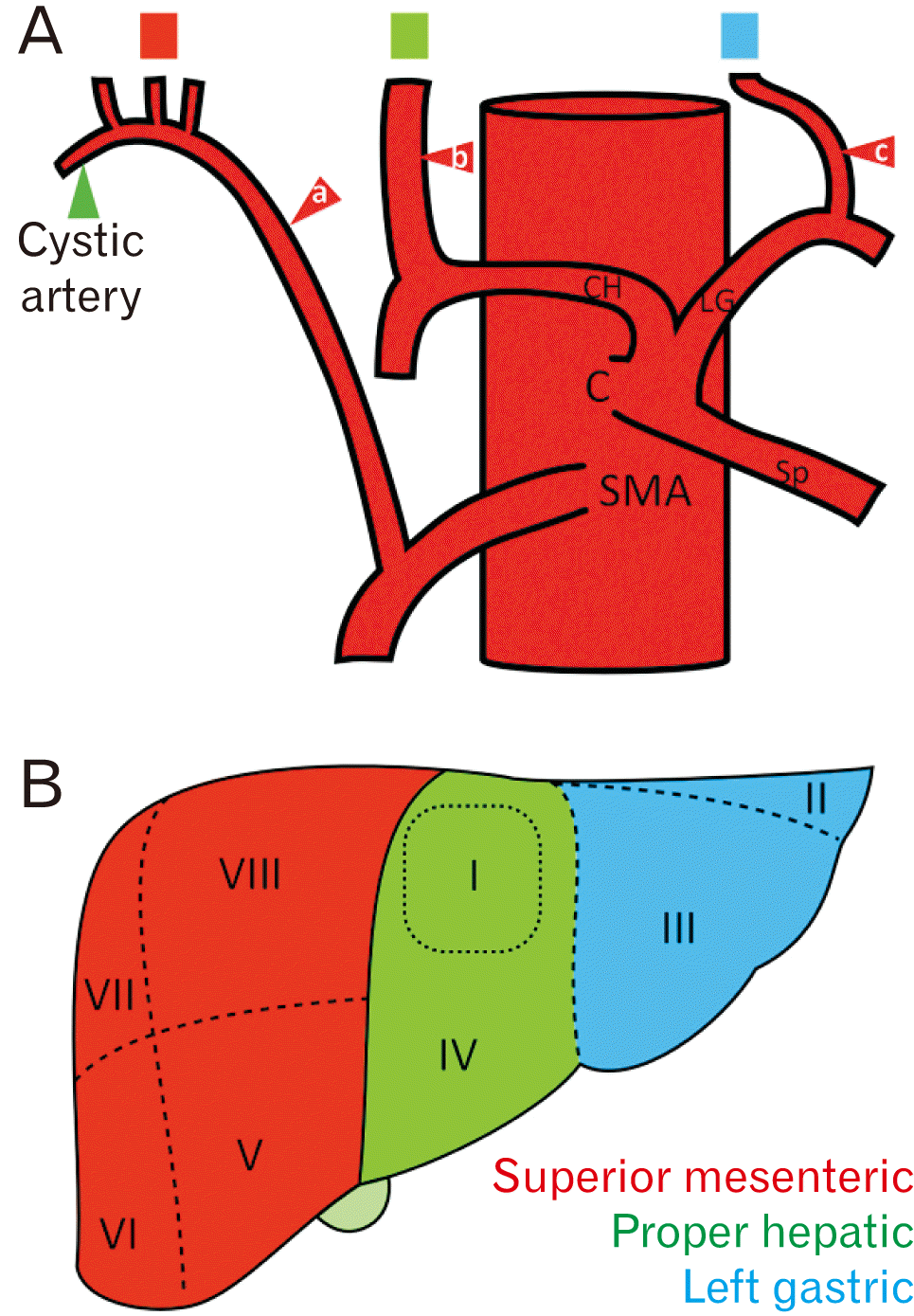
In the present case study, we describe an unusual pattern of hepatic blood supply. Three extrahepatic arteries arose from the superior mesenteric artery, proper hepatic artery, and LGA rather than the typical pattern of two extrahepatic arteries arising solely from the proper hepatic artery. The variation observed in this case study is a combination of Michels types II and III, and is classified as Michels type IV variation–both the right and left hepatic arteries are replaced with vessels originating from the SMA and LGA [
2]. The proper hepatic artery continues as the middle hepatic artery. This variation is rare and was found in 0.8% of individuals [
3]. As observed in
Fig. 1, the three extrahepatic arteries were similar in diameter suggesting substantial contributions from each, as indicated in
Fig. 2.
Variation in hepatic blood supply is common and may be traced to embryologic development. The liver begins developing during week 4 of gestation. It grows into the septum transversum, and by the end of week 6, the liver occupies most of the abdominal cavity. During the embryonic period the liver divides into three parts, each with its own primitive blood supply. A left lateral lobe is supplied by the left embryonic artery from the LGA. A central lobe is supplied by the middle embryonic artery from the CH. A right lateral lobe is supplied by the right embryonic artery originating from the omphalomesenteric artery, and it ascends posterior to the portal vein. The arteries are initially connected by a ventral longitudinal anastomosis, and if this fails to obliterate, a common celiomesenteric trunk may be observed. As the central lobe enlarges and overtakes the lateral lobes, the middle embryonic artery develops into the proper hepatic artery–this splits into left and right branches and becomes the predominant blood supply to the liver. The left and right embryonic arteries typically regress. Aberrant hepatic arteries may represent the persistence of embryologic hepatic arteries, or an incomplete obliteration of the ventral longitudinal anastomosis [
4]. In the current case study, the replaced left and right hepatic arteries appear in the location of the temporary embryonic vessels and may reflect a lack of regression of these arteries.
An understanding of hepatic arterial variation is important to prevent iatrogenic injury and surgical complications. Aberrant arteries may require a change to surgical procedures [
3]; therefore, preoperative imaging and knowledge of common variations are crucial [
5]. During liver procedures, all hepatic arteries must be preserved as each serves particular liver segments. There are surgical costs and benefits associated with hepatic arterial variations. In the current donor, the presence of three separate hepatic arteries may offer flexibility in resection of liver segments. However, it may require complex intraoperative reconstruction, increasing operative time and the likelihood of complications [
6]. A rLHA from the LGA can be damaged during procedures involving the lower esophagus or stomach, leading to hepatic necrosis. However, the aberrant vessel may provide collateral support in cases of porta hepatis obstruction [
7] and is likely to be unaffected in bile duct cancer [
8]. In the current donor, the middle hepatic artery supplies liver segment IV, which is often included in planes of resection. Understanding blood supply to this segment affects donor outcomes, liver regeneration, and the likelihood of liver ischemia [
9,
10]. A rRHA from the SMA can be injured or accidentally ligated in cholecystectomy [
11,
12] and pancreaticoduodenectomy [
13], compromising blood supply to the liver and bile duct. This increases risk of liver necrosis and breakdown of the hepatobiliary anastomosis, leading to biliary fistula. Occasionally, a rRHA travels through the head of the pancreas, increasing the likelihood of iatrogenic damage during resection [
14]. However, an rRHA may improve the prognosis for patients with cholangiocarcinoma as the vessel is farther away from the lesion and tends to be spared [
1]. A vessel branching from the SMA is also longer and reduces the likelihood of hepatic thrombosis following grafts in living liver donors [
15]. Overall, knowledge of hepatic artery variants is key to surgical planning and preventing intra- and post-operative complications.
In conclusion, this case study describes a rare variation of hepatic arterial supply discovered during routine dissection of the abdominal aorta and associated viscera. Embryonic development of the CT and SMA may contribute to a wide variety of aberrant hepatic arteries. Due to the relatively common occurrence, hepatic vasculature must be well understood to ensure successful outcomes during surgical procedures involving the liver.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download