Abstract
The abducens nerve (AN; cranial nerve VI) exits the brainstem at the inferior pontine sulcus, pierces the dura of the posterior cranial fossa, passes through the cavernous sinus in close contact to the internal carotid artery (ICA) and traverses the superior orbital fissure to reach the orbit to innervate the lateral rectus muscle. At its exit from the brainstem, the AN includes only axons from lower motor neurons in the abducens nucleus. However, as the AN crosses the ICA it receives a number of branches from the internal carotid sympathetic plexus. The arrangement, neurochemical profile and function of these sympathetic axons running along the AN remain unresolved. Herein, we use gross dissection and microscopic study of hematoxylin and eosin-stained sections and sections with tyrosine hydroxylase immunolabeling. Our results suggest the AN receives multiple bundles of unmyelinated axons that use norepinephrine as a neurotransmitter consistent with postganglionic sympathetic axons.
The abducens nerve (AN; cranial nerve VI) emerges from the brainstem near the inferior pontine sulcus where the pyramids emerge from the basilar pons [1]. As the AN leaves the brainstem it contains only axons from lower motor neurons in the abducens nucleus [1, 2]. From its exit, the AN courses superior and anteriorly, piercing the dura mater of the posterior cranial fossa along the posterior aspect of the sphenoid, lateral to the dorsum sellae. The AN then courses inferior to the petrosphenoidal ligament in Dorello’s canal, and enters the cavernous sinus [2]. Within the cavernous sinus, the AN courses along the lateral aspect of the internal carotid artery (ICA) and receives communicating branches from the internal carotid sympathetic plexus (ICP) and the ophthalmic division of the trigeminal nerve [2, 3]. This contribution from the ICP to the AN has been subject to some controversy. An interesting account is provided by Johnston and Parkinson [4]. These authors note that contributions of the ICP to the AN was described in Quain and Sharpey’s text [5] and a very similar description appeared later in Gray’s [6]. The description in Gray’s was considered plagiarism [7] and was subsequently removed for a number of editions.
While a sympathetic contribution to the AN has been known for many years, and that space-occupying lesions in the cavernous sinus can result in an AN palsy and partial Horner’s syndrome, few studies have provided any further characterization of this connection or examined function or termination of such sympathetic axons. McGrath found that branches from the ICP intermingle with somatic axons of the AN [8]. Other studies found axons from the ICP coursing alongside the somatic axons of the AN, and specifically noted to not intermingle with AN fibers; at least some of these axons leave the AN in the orbit to join branches from the trigeminal nerve [4, 9, 10]. More recently, Iwanaga et al. [11] found that the ICP provides branches to the AN in 94.4% of dissections and this ranges from 1–3 branches; they however found no gross evidence of ganglia along the AN. Wysiadecki et al. [12] showed small axon fascicles outside the main trunk of the AN. Together, these histological studies of the cavernous segment of the AN yields some inconsistencies about arrangement of nerve fascicles but also fail to demonstrate that these axons are visceral in nature. One characteristic feature of postganglionic sympathetic neurons is the utilization of norepinephrine as a neurotransmitter. Such neurons can be identified by the presence of tyrosine hydroxylase (TH) using immunohistochemistry [13]. Herein, we aimed to further characterize the distribution of axons from the ICP along the AN using gross dissection, routine histology and immunolabeling for TH.
The ICA and AN were approached in the cavernous sinus in a total of six subjects (Table 1). In one subject, the cavernous sinus was approached from the anterior and medial aspect and the ICA was exposed from the skull base to its exit from the cavernous sinus and its termination into the anterior and middle cerebral arteries. In this same subject, the AN was traced from the brainstem all the way to the superior orbital fissure. In the remaining five subjects the cavernous sinus was approached laterally from the middle cranial fossa. In three of these dissections, a portion of the AN was removed (distal to the nerve receiving contributions from the ICP). This segment of nerve was postfixed in 10% formalin in water. Nerve blocks to be used for histology were embedded in paraffin and processed for hematoxylin and eosin (H&E) staining. Additional tissue blocks from two ANs were processed for immunohistochemistry for TH. TH is an enzyme important in the biosynthesis of norepinephrine and is found in cell bodies and axons of neurons using norepinephrine as a neurotransmitter. These blocks were cryoprotected in 30% sucrose in phosphate buffered saline (PBS, pH 7.4). Nerve blocks were sectioned in the coronal plane on a freezing-stage microtome and a thickness of 20 μm. Free-floating tissue sections were rinsed in PBS, blocked for 1 hour in 1% normal horse serum and 0.5% triton-X. Sections were incubated overnight in primary antiserum (rabbit anti-TH [1:1,000; Abcam ab112]) with 1% normal horse serum. Sections were rinsed in PBS, incubated in goat anti-rabbit DyLight 488 (Vector Labs) for 2 hours, rinsed and counterstained with neurotrace red (NT; a red fluorescent counterstain). Sections were mounted onto glass slides from cresyl gelatin and coverslipped with Entellan. Photomicrographs were taken with a DP71 digital camera (Olympus) on an Olympus CKX41 microscope (Olympus).
In each of the six subjects in this study, the cavernous segment of the AN received 2–4 branches from the ICP. Images from the anterior-medial dissection are shown in Fig. 1A, B. The ICA is viewed from the medial aspect; the optic nerve (CN II), oculomotor nerve (CN III) and AN (white arrowheads) are seen near the ICA. The AN received branches from the ICP (blue arrowheads); these branches from the ICP joined the inferior aspect of the AN along the caudal aspect of the cavernous sinus (Fig. 1C). In the six specimens examined, the number of branches from the ICP joining the AN varied: in 3 subjects there were 2 branches from the ICP, in 2 subjects there were 3 branches (Fig. 1B, C) and in 1 subject there were 4 branches. Contributions from the ICP were not always uniform in size; there was usually a dominant branch (see asterisk in Fig. 1C).
Microscopic examination of H&E-stained sections revealed that the large core bundle of the AN was composed of myelinated axons (Fig. 2A, asterisks; Fig. 2B, black arrows) surrounded by layers of connective tissue. There were however numerous bundles of unmyelinated axons situated outside the core AN bundles, but within the same connective tissue sleeve. The section in Fig. 2A includes seven distinct bundles (black arrowheads) coursing parallel to the AN fibers. The number of unmyelinated bundles within the AN varied in the three specimens examined, ranging from 4 to 7 bundles. Fig. 2C shows a higher magnification view of one of the bundles of unmyelinated axons. These bundles frequently included a small number of myelinated axons (Fig. 2C, black arrows). In two of the three specimens studied microscopically, there were clusters of large, irregular somata with eccentric nuclei, finely granular Nissl substance and lipofuscin; satellite and supportive cells did not form a capsule around the cell bodies (Fig. 2D, yellow arrows). These neuronal cell bodies were intermingled with unmyelinated axons (blue asterisk).
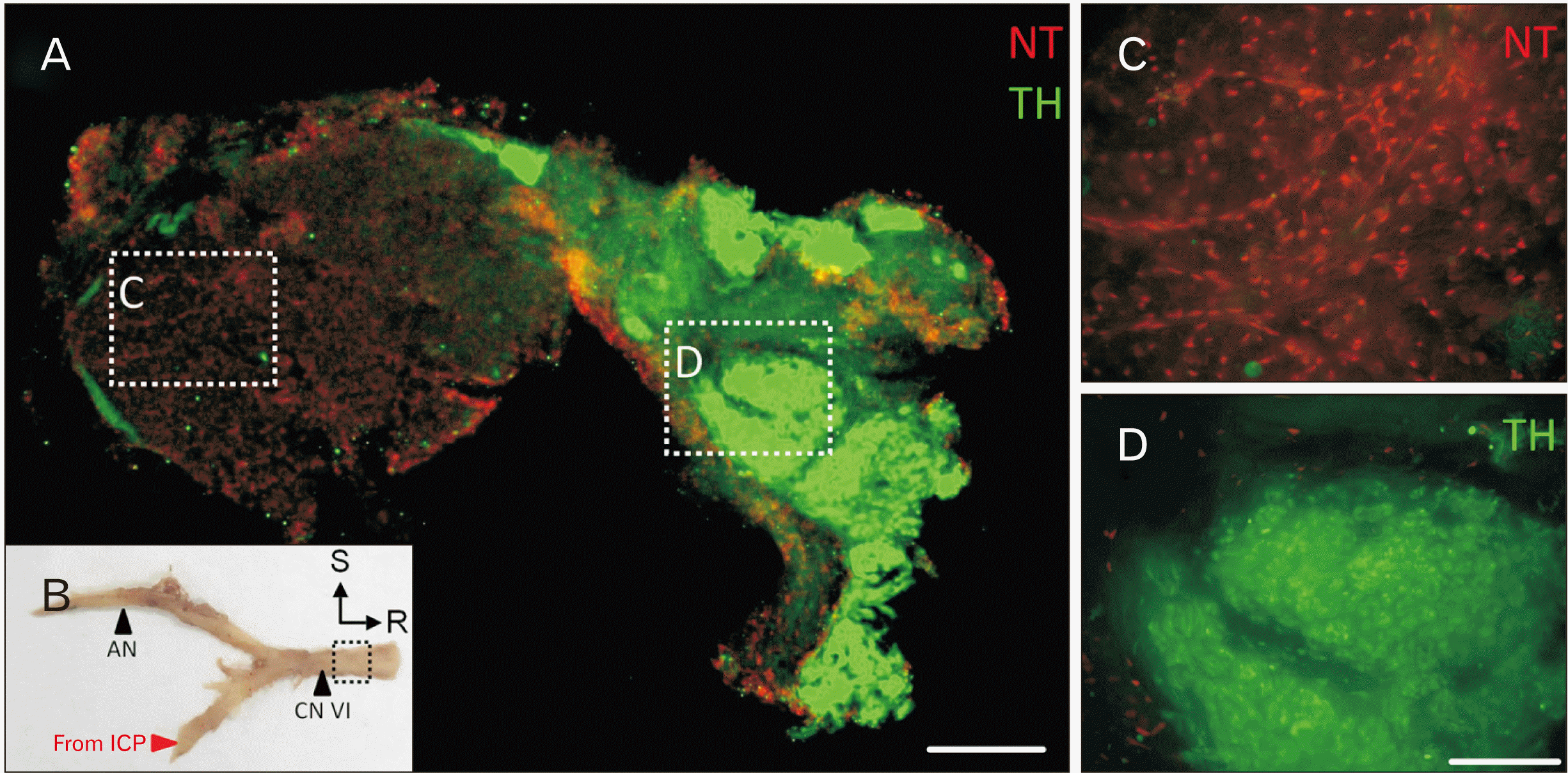
Immunolabeling of frozen sections of the AN distal to receiving branches from the ICP revealed distinct axon bundles that were TH-positive (Fig. 3A, green). Axons in the core bundles of the AN were negative for TH (Fig. 3C). TH-immunolabeled axons bundles were found at the periphery of the AN and found in small clusters - consistent with the location of unmyelinated axons in H&E-stained tissue sections.
The results from this study support previous reports that demonstrate the AN receiving branches from the ICP within the cavernous sinus. Our findings indicate that axons from the ICP course along the main nerve bundles of the AN in up to seven distinct bundles, that these axons from the ICP are largely unmyelinated and are noradrenergic. The few myelinated axons found in these bundles are likely to be visceral afferent axons arising from dorsal root ganglia at T1-4. Regardless, the majority of these axons from the ICP are TH+ and most likely use norepinephrine as a neurotransmitter, consistent with postganglionic sympathetic axons.
Ganglia have been described previously in the cavernous sinus, but were interpreted to be parasympathetic [14, 15]. Consistent with this interpretation, Bleys et al. [10] found ganglia associated with cholinergic axons. However, the ganglia that we found were along the course of the AN, within the epineurium and in close proximity to unmyelinated, noradrenergic axons and are therefore most likely sympathetic.
Our study of H&E-stained sections indicate that the axons from the ICP make up a significant portion of the cavernous portion of the AN. Previous reports suggested that the majority of these axons leave the AN in the orbit to join branches of the trigeminal nerve, although some continue along the AN [9]. These sympathetic axons are potentially destined for numerous structures in the orbit including the lacrimal gland, branches of the ophthalmic artery, superior tarsal muscle, skin of the eyelid or forehead or pupillary and ciliary muscles in the eye. The target of these sympathetic axons in the AN is of particular interest. If these axons routinely or occasionally innervate the superior tarsal muscle and/or dilator pupillae muscle, it is possible that injury to the orbital segment of the AN could result in diplopia and ptosis or miosis.
Acknowledgements
The authors are grateful to those who donated their bodies to medical education and anatomical research. Results from such anatomical research provides an important avenue to extend our understanding of human structure and function and improve patient care.
Notes
References
1. Standring S. 2016. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. Elsevier;DOI: 10.1002/ca.22677.
2. Cunningham DJ, Robinson A. 1931. Cunningham's text-book of anatomy. 6th ed. Oxford University Press;DOI: 10.5962/bhl.title.44024.
3. Monro A. 1746. The anatomy of the human bones and nerves. 4th ed. Hamilton and Balfour;DOI: 10.5962/bhl.title.114861.
4. Johnston JA, Parkinson D. 1974; Intracranial sympathetic pathways associated with the sixth cranial nerve. J Neurosurg. 40:236–43. DOI: 10.3171/jns.1974.40.2.0236. PMID: 4809122.

5. Quain J, Leidy J, Quain R, Sharpey W. 1849. Human anatomy. 1st ed. Vol 2:Lea and Blanchard;p. 343–4.
6. Gray H, Pick TP, Howden R. 1901. Anatomy, descriptive and surgical. 15th ed. Lea Brothers;p. 802. DOI: 10.5962/bhl.title.31366.
7. Plarr V, Power D, Spencer WG, Gask GE. 1930. Plarr's lives of the fellows of the Royal College of Surgeons of England. Vol 1:John Wright & Sons;p. 464.
8. McGrath P. 1977; The cavernous sinus: an anatomical survey. Aust N Z J Surg. 47:601–13. DOI: 10.1111/j.1445-2197.1977.tb06591.x. PMID: 273404.

9. Parkinson D, Johnston J, Chaudhuri A. 1978; Sympathetic connections to the fifth and sixth cranial nerves. Anat Rec. 191:221–6. DOI: 10.1002/ar.1091910207. PMID: 666018.

10. Bleys RL, Janssen LM, Groen GJ. 2001; The lateral sellar nerve plexus and its connections in humans. J Neurosurg. 95:102–10. DOI: 10.3171/jns.2001.95.1.0102. PMID: 11453377.

11. Iwanaga J, Anand MK, Camacho A, Rodriguez F, Watson C, Caskey EL, Dumont AS, Tubbs RS. 2020; Surgical anatomy of the internal carotid plexus branches to the abducens nerve in the cavernous sinus. Clin Neurol Neurosurg. 191:105690. DOI: 10.1016/j.clineuro.2020.105690. PMID: 31982693.

12. Wysiadecki G, Radek M, Tubbs RS, Iwanaga J, Walocha J, Brzeziński P, Polguj M. 2021; Gross and micro-anatomical study of the cavernous segment of the abducens nerve and its relationships to internal carotid plexus: application to skull base surgery. Brain Sci. 11:649. DOI: 10.3390/brainsci11050649. PMID: 34065668. PMCID: PMC8156379. PMID: 7c47d66c3189421a831c3e0d82f96484.

13. Pickel VM, Joh TH, Reis DJ. 1975; Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc Natl Acad Sci U S A. 72:659–63. DOI: 10.1073/pnas.72.2.659. PMID: 235760. PMCID: PMC432374.

14. Gellért A. 1934; Ganglia of the internal carotid plexus. J Anat. 68(Pt 3):318–22. PMID: 17104480. PMCID: PMC1249031.
15. Carvalho VC. 1985; Nerve cells in the human cavernous sinus. Anat Anz. 159:29–32. PMID: 4096407.
Fig. 1
Contribution from the ICP to the AN. (A, B) are different stages of the dissection of the right ICA and cavernous sinus of a single specimen. (A) The ICA has been exposed from the anterior aspect and the cavernous sinus has been dissected away. The optic nerve (CN II), oculomotor nerve (CN III) and AN are visible. The AN (white arrowheads) can be seen emerging from the brainstem and coursing lateral to the ICA. Branches from the ICP joining the AN are indicated by blue arrowheads. A deeper dissection of this region is (B) the AN (white arrowheads) is pulled away from the ICA with forceps to demonstrate the branches from the ICP (blue arrowheads). (C) is a dissection of the ICA and cavernous sinus from the left side of a different specimen where the venous plexus, CN II and branches for CN V have been removed. The AN (white arrowheads) courses lateral to the ICA and is joined by multiple branches from the ICP (blue arrowheads). The scale bars are all equal to 5 mm. ICP, internal carotid sympathetic plexus; AN, abducens nerve; ICA, internal carotid artery; TT, torus tubaris, V, vertebral artery; MO, medulla oblongata; P, pons; B, basilar artery; R, rostral; S, superior.
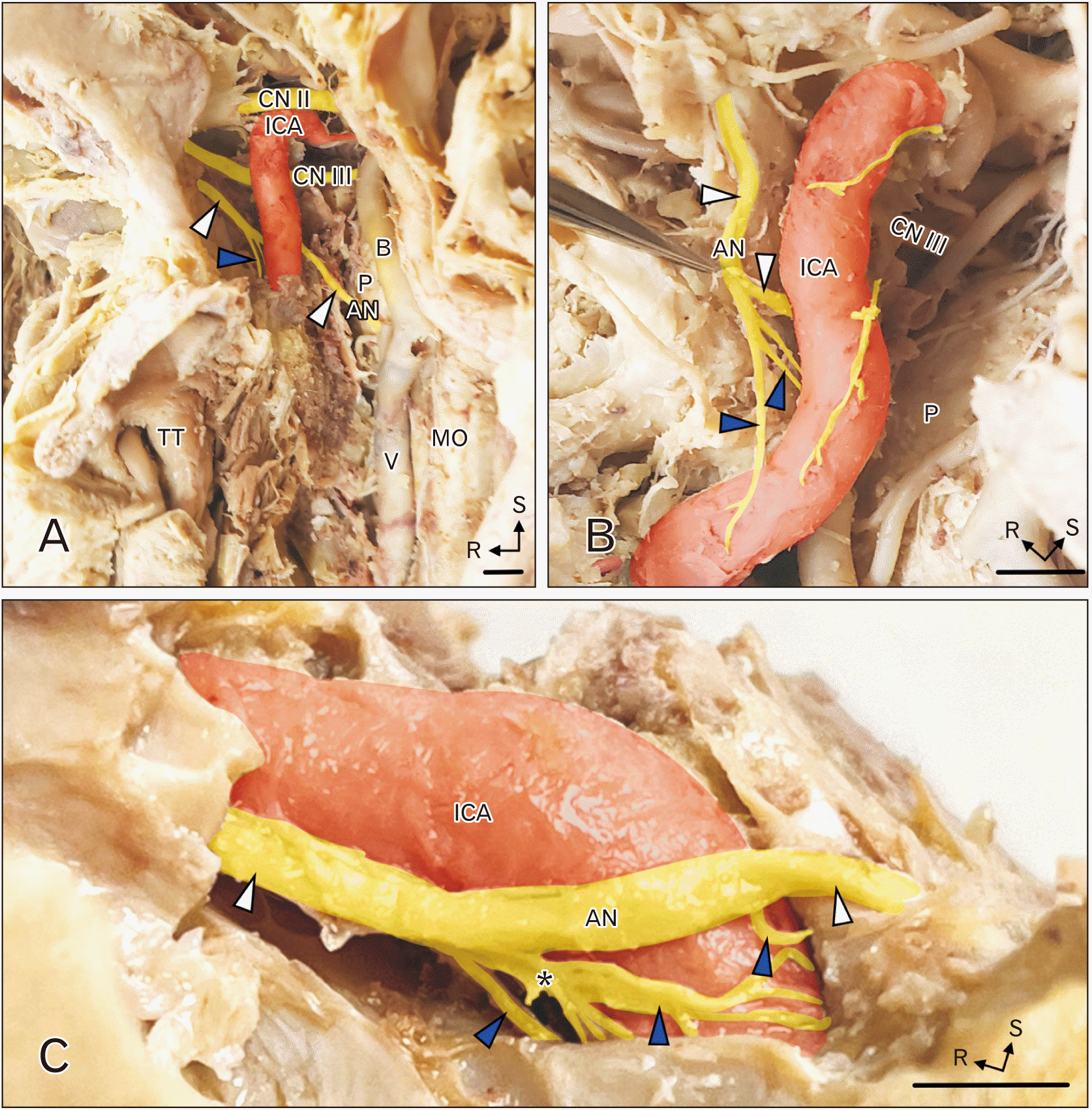
Fig. 2
Unmyelinated axons along the AN. (A) is a low magnification image of an H&E-stained section through the cavernous segment of the AN. The fascicles indicated by black asterisks are composed of myelinated axons; the fascicles indicated by the black arrowheads are largely composed of unmyelinated axons. (B) shows a higher magnification view of the region indicated by the black dashed box. Individual myelinated axons are indicated by the black arrows. (C) shows a higher magnification view of the region indicated by the blue dashed box. This bundle of axons (blue asterisk) contains mainly unmyelinated axons; myelinated axons are indicated by the black arrows. (D) shows a higher magnification view of the region indicated by the red dashed box. This fascicle contains only unmyelinated axons and a collection of neuronal cell bodies (yellow arrows). The scale bar in A is equal to 300 mm; the scale bar in D is equal to 40 mm and applies to B–D. AN, abducens nerve.
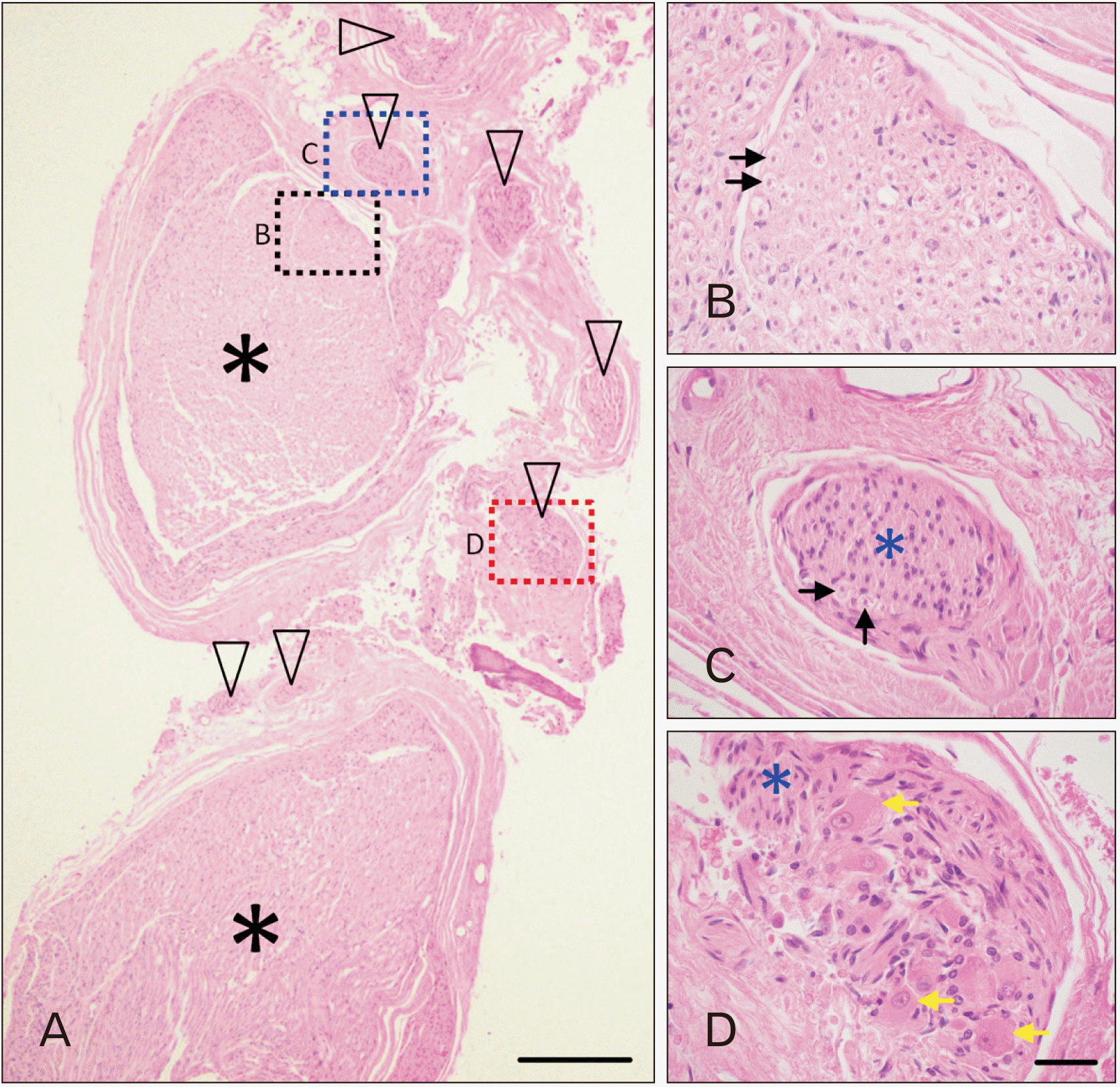
Fig. 3
TH+ axons along the AN. (A) shows a cross section of the AN labeled for TH and counterstained with neurotrace red (NT). This section is taken from the AN distal to receiving branches from the ICP (boxed area in B). A higher magnification view of the main trunk of the AN is (C); there is no TH immunolabeling in this part of the nerve. A higher magnification view of the peripheral aspect of the nerve is (D); the majority of these axons are TH+, consistent with noradrenergic function. The scale bar in A is equal to 200 mm; the scale bar in D is equal to 40 mm and applies to both C and D. AN, abducens nerve; S, superior; R, rostral; ICP, internal carotid sympathetic plexus.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download