Introduction
Materials and Methods
Table 1
| Size of fetus | Joint cartilage | Cavitation | Ossification of ilium |
|---|---|---|---|
| 49 mm CRL | None | None | Anterior side of the future joint |
| 52 mm (Fig. 1A) | None | None | Anterior side of the future joint |
| 56 mm | None | None | Anterior side of the future joint |
| 62 mm | None | None | Anterior side of the future joint |
| 76 mm (Fig. 1B) | None | None | Reached the future jointb |
| 80 mm | None | None | Reached the future joint |
| 82 mm (Fig. 1C) | Small | None | Reached the future jointb |
| 85 mm | None | None | Reached the future joint |
| 90 mm (Fig. 1D) | Extended anterior 1/3a | None | Covered the future joint |
| 92 mm | Small | None | Covered the future joint |
| 97 mm | Extended anterior 1/3 | None | Covered the future joint |
| 97 mm-2 | Small | None | Covered the future joint |
| 103 mm | Extended anterior 1/3 | None | Covered the future joint |
| 107 mm | Small | None | Covered the future joint |
| 115 mm | Extended anterior 1/3 | None | Covered the future joint |
| 120 mm (Fig. 2A) | Extended anterior 1/4 | None | Covered the future joint |
| 145 mm (Fig. 2B) | Extended almost halfa | Present | Extended over the joint |
| 150 mm | Extended almost half | Present | Extended over the joint |
| 155 mm (Fig. 2C) | Extended almost half | None | Covered the future joint |
| 160 mm | Extended almost half | None | Covered the future joint |
| 165 mm | Extended anterior 2/3 | Present | Covered the future joint |
| 210 mm | Fully extended | Present | Extended over the joint |
| 232 mm (Fig. 2D) | Fully extended | Present | Extended over the joint |
| 250 mm | Fully extended | Present | Extended over the joint |
aThe joint cartilage of the ilium extended along the bony ilium from the anterior end of the future joint area and covered the anterior 1/3 (or the anterior half) of the area. bOssification of the ilium started at the far anterior side of the future joint, extended posteriorly to reach the future joint area and, further extended over the area.
Results
Observations of the SIJ
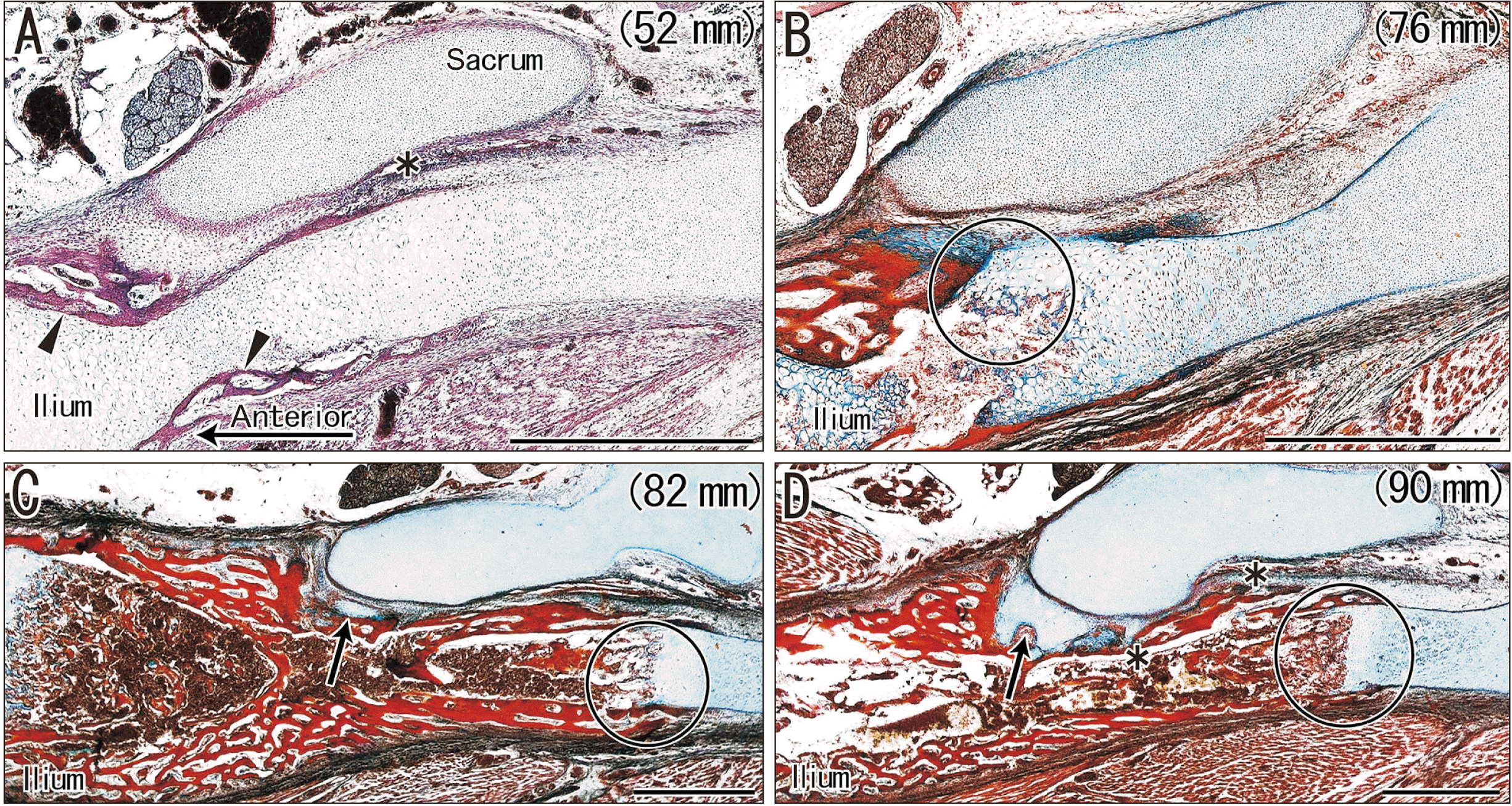 | Fig. 1Horizontal sections of sacroiliac joints in four fetuses (crown-rump length: 52–90 mm) that were stained with H&E (A) or azan (B–D). (A, B) show that ossification of the ilium occurs in the anterior part, but does not reach the future joint area. (A) shows membranous bones (arrowheads), not cartilaginous bones. (C) shows a small cartilage mass attached to the ilium (arrow). (D) shows that the cartilage covers almost one-third of the future joint area (arrow). The circles in (B–D) indicate endochondral ossification of the ilium. The asterisks in (A, D) indicate an artifact space produced during histological procedure. (A–D) Scale bars=1 mm. |
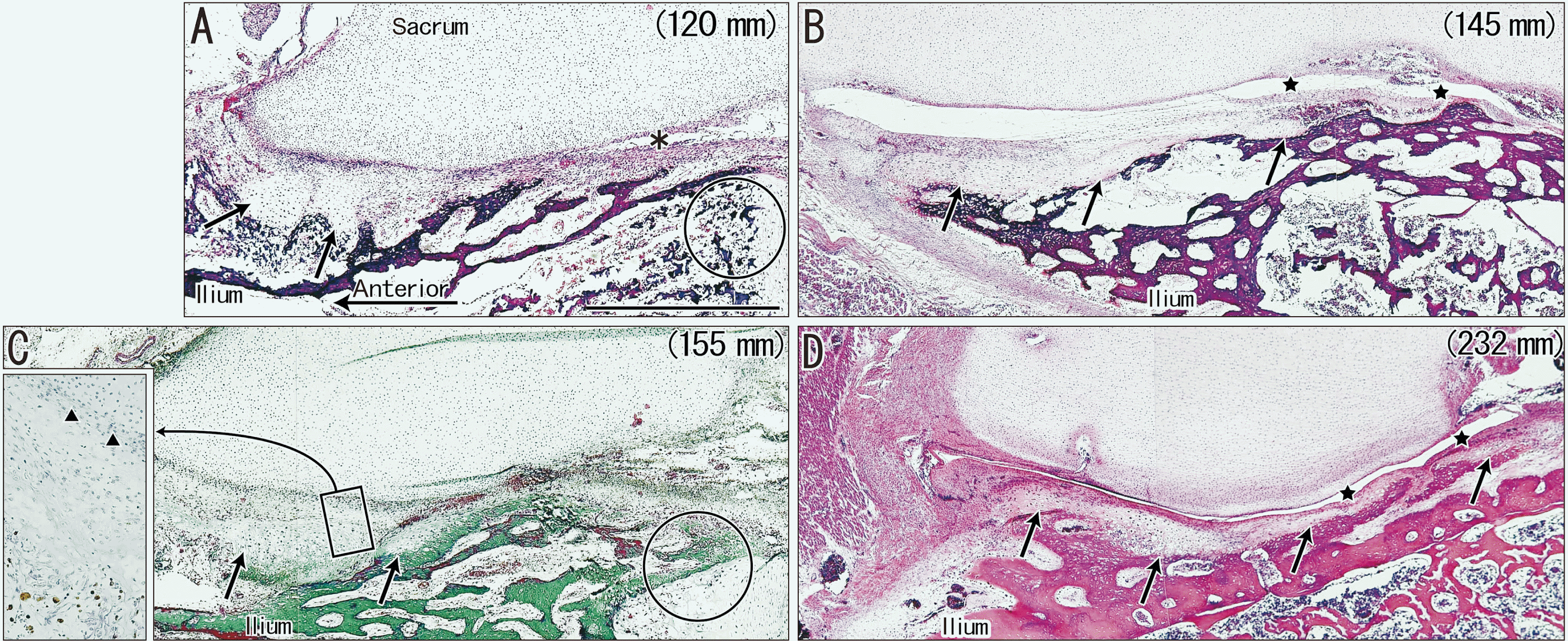 | Fig. 2Horizontal sections of sacroiliac joints in four fetuses (crown-rump length: 120–232 mm) that were stained with H&E (A, B, D) or Masson’s trichrome (C). All panels show the bony ilium covering the joint area. (A–C) show that the joint cartilage of the ilium (arrows) covers the anterior half of the joint area. (D) shows the cartilage extends posteriorly over the joint. (B, D) show the joint cavities (stars). The insert on the left of (C) (corresponding to the rectangle in the main panel) shows CD68-positive cells at higher magnification, in which the future joint space (triangles) contains no macrophages. The circles in (A, C) indicate endochondral ossification of the ilium. The asterisk in (A) indicates an artifact space produced during the histological procedure. All panels (except the C insert) were at the same magnification. (A) Scale bar=1 mm. |
Observations of the TMJ and humeroradial joint
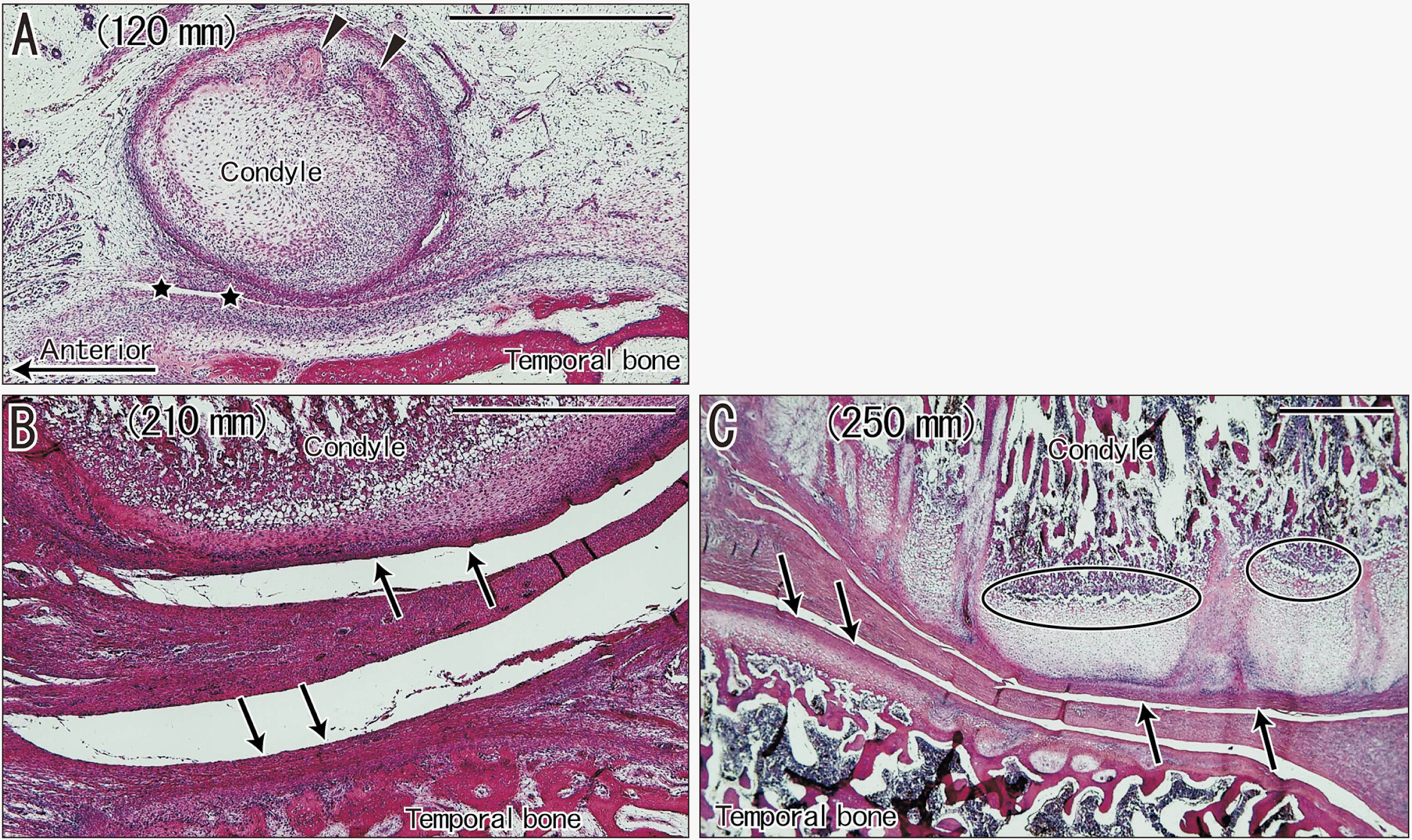 | Fig. 3Sagittal sections of temporomandibular joints in three fetuses (crown- rump length: 125, 210, and 250 mm) that were stained with H&E. (A) shows attachment of the condylar cartilage to the bony temporal bone, with initiation of ossification in the condyle (arrowheads) and cavitation (stars). (B, C) show that the joint cavity is large and the disk is thick, but a periosteum-like membrane (arrows) covers the joint surface of the condyle and temporal bone, instead of the joint cartilage. Ovals in (C) show endochondral ossification occurs in the condyle. (A–C) Scale bars=1 mm. |
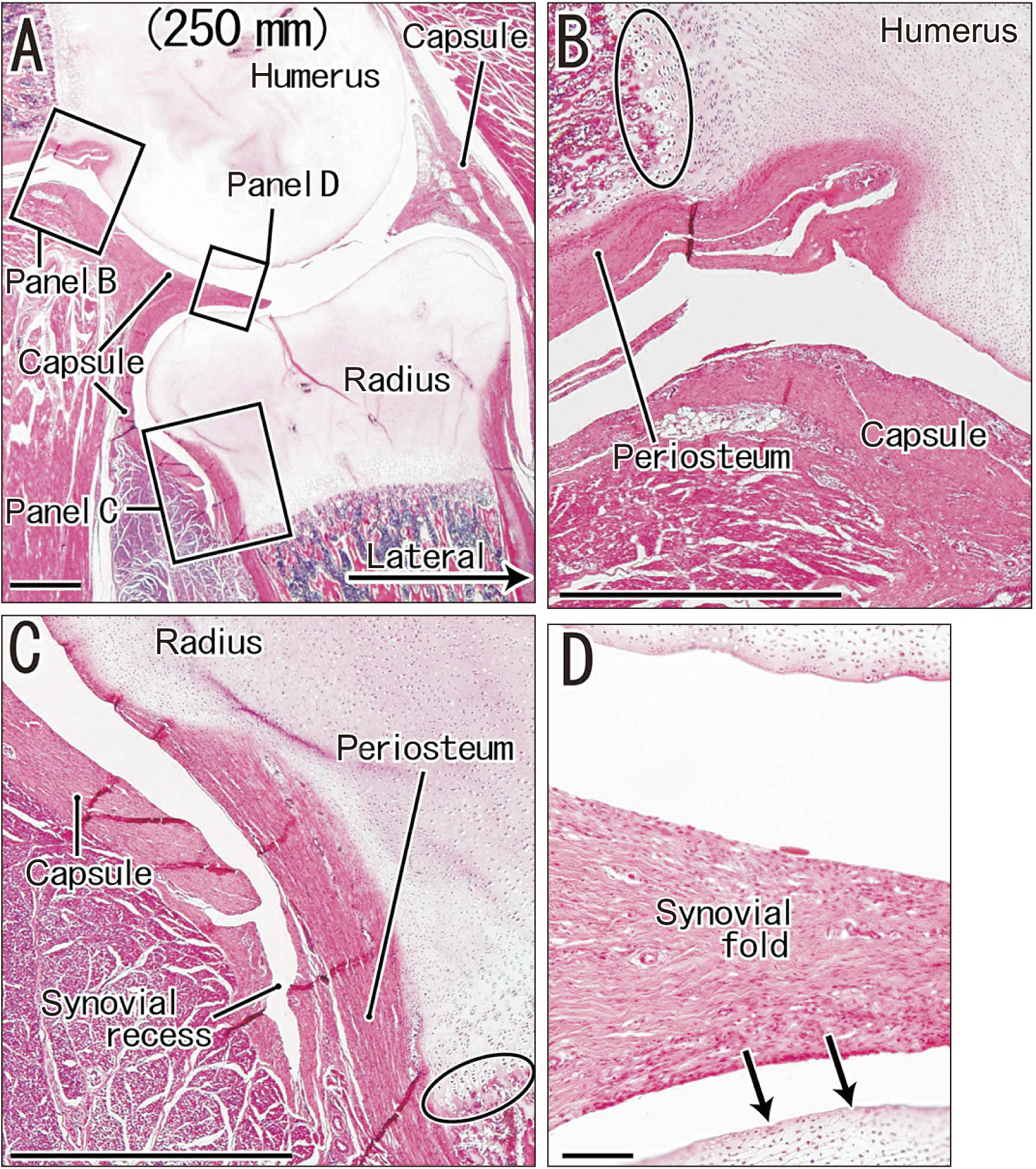 | Fig. 4Sagittal sections of the humeroradial joint in a fetus (crown-rump length: 250 mm) that were stained with H&E. (A) shows the topographical anatomy around the humeroradial joint. (B–D) are higher magnification views of three rectangles in (A). (B, C) show the periosteum and joint capsule at recesses of the joint cavity. (D) shows a thick synovial fold and its adjacent joint cartilage, and that the periosteum does not continue to the joint surface in the humeroradial joint (arrows). The oval in (B) shows the endochondral ossification is at the distal end of the periosteum in the humerus, and the oval in (C) shows it is covered by the periosteum in the radius. (A) Scale bar=1 mm; (B–D) Scale bars=0.1 mm. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download