Abstract
Adult neurogenesis has been reported in the hypothalamus, subventricular zone and subgranular zone in the hippocamp. Recent studies indicated that new cells in the hypothalamus are affected by diet. We previously showed beneficial effects of safflower seed oil (SSO), a rich source of linoleic acid (LA; 74%), on proliferation and differentiation of neural stem cells (NSCs) in vitro. In this study, the effect of SSO on hypothalamic neurogenesis was investigated in vivo, in comparison to synthetic LA. Adult mice were treated with SSO (400 mg/kg) and pure synthetic LA (300 mg/kg), at similar concentrations of LA, for 8 weeks and then hypothalamic NSCs were cultured and subsequently used for Neurosphere-forming assay. In addition, serum levels of brain-derived neurotrophic factor (BNDF) were measured using enzyme-linked immunosorbent assay. Administration of SSO for 8 weeks in adult mice promoted the proliferation of NSCs isolated from SSO-treated mice. Immunofluorescence staining of the hypothalamus showed that the frequency of astrocytes (glial fibrillary acidic protein+ cells) are not affected by LA or SSO. However, the frequency of immature (doublecortin+ cells) and mature (neuronal nuclei+ cells) neurons significantly increased in LA- and SSO-treated mice, compared to vehicle. Furthermore, both LA and SSO caused a significant increase in the serum levels of BDNF. Importantly, SSO acted more potently than LA in all experiments. The presence of other fatty acids in SSO, such as oleic acid and palmitic acid, suggests that they could be responsible for SSO positive effect on hypothalamic proliferation and neurogenesis, compared to synthetic LA at similar concentrations.
Neurogenesis is a highly regulated process that leads to the production of new neurons. Adult mammalian neurogenesis mainly involves the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles [1, 2]. In addition, it has been reported that it can occur in the basal forebrain [3], amygdala [4], subcortical white matter [5], and the hypothalamus [6]. Moreover, it has been suggested that neural progenitor cells (NPCs) in the hypothalamus may represent self-renewing cells that give rise to new tanycytes, astrocytes, and neurons [7]. On the other hand, the decline in neurogenesis has been linked to the progression of neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, physical wave and others [8]. These disorders are characterized by the loss of neurons in specific brain regions [9], whereas stimulation of neurogenesis is one of the strategies suggested to help functional recovery [10].
The use of dietary factors to induce neurogenesis is considered a promising therapeutic approach in neuroscience [11]. It is much easier and practical to stimulate neurogenesis by alteration of exogenous factors, such as diet, than by manipulating endogenous factors, such as genetic networks. Among dietary agents, polyunsaturated fatty acids (PUFAs) have been suggested as critical nutritional factors for proper neural development and function [12, 13]. It has been known for a long time that PUFAs are produced in plants and are not synthesized in vertebrates [14]. Linoleic acid (LA, C18:2n-6) is the main n-6 PUFA found in plant oils, such as soybean and corn, and which are extensively used in western diets [15]. In our previous in vitro study, we demonstrated that Safflower (Carthamus tinctorius L.) seed oil (SSO) is a rich source of LA (73.64%) that affected the proliferative and differentiative capacities of neural stem cells (NSCs) and increased the number of neurons (β-III tubulin positive cells). In contrast, synthetic LA, at similar concentrations to natural SSO LA, could not affect the number of neurons [16].
In this study, we investigated whether natural LA of SSO, in comparison to synthetic LA, could induce hypothalamic neurogenesis in vivo, when administered orally to mice. In addition, the potential increase in hypothalamic neurogenesis was then tested for its association with brain-derived neurotrophic factor (BDNF) serum levels.
Young adult C57BL/6J mice (8–10 weeks old, 30–35 g) were used in this study. The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and Ethics Committees of the University of Yasuj, which conforms to the provisions of the Declaration of Helsinki (as revised in Brazil in 2013). Animals had free access to food and water and were housed under pathogen- free conditions.
The safflower seed oil species chosen were Carthamus. Tinctorius (genotype: C4110), identical to that used previously [17]. Chemically, this seed oil contains 73.64% LA, 15.14% oleic acid (OA), 5.7% palmitic acid (PA), and a total of 2.15% myristic (C14:0), palmitoleic (C16:1), stearic (C18:0), arachidic (C20:0), and behenic (C22:0) acids.
Mice were divided into four groups (n=10 per group) as follows: (i) control mice (Ctrl); (ii) vehicle mice (Vehicle), received an equal volume of solution containing 0.5% DMSO solvent (iii) synthetic LA-treated mice (300 mg/kg) and (iv) SSO-treated mice (407.4 mg/kg). All treatments were done orally for eight weeks.
Isolation of hypothalamic NSCs was performed as described previously [18]. Briefly, the hypothalamus was micro-dissected from adult mice (7 mice per group) on day 21 of the study under sterile. Hypothalamic pieces were then mechanically disrupted into single cells by repeated pipetting in serum-free neurosphere N2 medium. The desired cell population was suspended in a growth medium containing DMEM/F12 (1:1), 0.6% (w/v) glucose, 0.1125% (w/v) sodium bicarbonate, 2 mM L-glutamine, 5 mM HEPES, 100 μg/ml human apo transferrin, 20 nM progesterone, 30 nM sodium selenite, 60 μM putrescine, and 25 μg/ml insulin. Cells were then seeded in T25 flasks in suspension at a density of 1×105 cells/ml in a proliferation medium consisting of the above N2 medium supplemented with 20 ng/ml basic fibroblast growth factor (bFGF; R&D Systems) and 2 mg/ml heparin (Sigma-Aldrich). Cells were maintained in an incubator with a humidified atmosphere containing 5% CO2 at 37°C for 7 days. Neurospheres were then harvested by centrifugation, dissociated using trypsin and EDTA (Sigma-Aldrich), and reseeded for the following experiments.
Neurosphere-forming cells obtained from passage-1 flasks were then dissociated into single cells using 0.05% trypsin-EDTA (Sigma-Aldrich), and reseeded for the following experiments after determining the cell density, using trypan blue exclusion assay. Cells were then cultured at 25 cells/μl in 0.2 ml media in uncoated well plates. The total number of neurospheres, with a diameter of >50 μm, was counted after 5 days using an Olympus inverted light microscope and expressed as the neurosphere-forming frequency per well.
At the end of the study, mice (7 per group on day 21) were deeply anesthetized with ketamine/xylazine (5/1) and then intracardially perfused with phosphate-buffered saline (PBS), followed by 3.7% paraformaldehyde in PBS (0.1 M). Brains were carefully harvested and placed in the same fixative overnight. Fixed tissues were then paraffin-embedded and cut as 6 μm sections.
Fixed tissue sections were performed by standard histochemistry method against anti-glial fibrillary acidic protein (GFAP), anti-neuronal nuclei (NeuN), or anti-doublecortin (DCX) antibodies, and then incubated overnight at 4°C. The next day, sections were incubated for 1 hours with appropriate secondary antibody. Representative pictures for each mouse were then taken using a fluorescent microscope (Olympus IX-71) equipped with a Canon EOS digital camera. Cell counts were performed (4 fields/section, 4 sections/mouse for a total of 16 fields/mouse) and data was presented as the mean frequency of positive cells in each group.
Simultaneously with the isolation of NSCs on day 21, then Serum was obtained by centrifugation of blood sample at 2,500 rpm for 10 minutes and then frozen at −80°C until ELISA was performed. Serum levels of BDNF were measured using an ELISA Kit according to the manufacturer’s protocol (Sigma Aldrich).
Results are presented as the mean with error bars indicating the standard error of the mean (Mean±SEM). GraphPad Prism (Version 6.01) software was used to perform statistical analyses. Following the assessment of normality, ordinary one-way ANOVA followed by Tukey post-hoc test was used to analyze the data.
The effect of SSO, compared to synthetic LA, on NSC proliferation was evaluated using the neurosphere assay (Fig. 1A). Neurosphere formation reflects the self-renewal capacity of NSCs when they are plated at a very low density. In this study, NSCs formed neurospheres of various sizes with diameters ranging between 50 μm to >100 μm (Fig. 1B). Results showed that SSO administration, but not synthetic LA, caused a significant (**P<0.01) increase in the number of neurospheres, in comparison to vehicle treatment, respectively (85.8±6.4 vs. 72.4± 4.5 vs. 59.2±4.1; Fig. 1B). Moreover, not only SSO but also synthetic LA demonstrated a significant (**P<0.01 vs. *P<0.05) increase in the number of single cells obtained from neurospheres, compared to vehicle (Fig. 1C).
To examine the role of SSO on neural development in vivo, hypothalamic astrocytes and neurons were identified using immunofluorescence analysis following oral SSO administration in mice for 8 weeks. First, astrocytes were examined using an antibody against GFAP (Fig. 2A) and the number of GFAP positive cells were counted (Fig. 2B). Results showed that SSO, but also synthetic LA, caused a slight but insignificant increase in the frequency of GFAP positive cells, in comparison to vehicle treated mice (Fig. 2B). On the other hand, mature and immature neurons were examined using antibodies against NeuN and protein DCX, respectively (Figs. 3A, 4A). Quantification of the number of NeuN positive cells showed that SSO, but also synthetic LA, caused a significant (****P<0.0001 vs. *P<0.05) increase in the frequency of cells that were positively labeled for mature neurons (Fig. 3B), in comparison to the vehicle group. It is important to note that the increase in neuron frequency due to natural SSO was significantly (*P<0.05) more potent than that of synthetic LA. Furthermore, both SSO and synthetic LA significantly (**P<0.01 vs. *P<0.05) increased the number of immature neurons, as shown by the increase in DCX positive cells (Fig. 4B), in comparison to the vehicle group. This increase in immature neurons by SSO or LA was nearly doubled (SSO: 15 vs. LA: 12 vs. Vehicle: 8, per section respectively), compared to the modest increase of mature neurons (SSO: 920 vs. LA: 812 vs. Vehicle: 750, respectively) (Figs. 3B, 4B).
Taken together, these data indicate that neither LA nor SSO can alter the number of astrocytes; however, they can both significantly increase the frequency of mature and immature neurons, with SSO having a significantly higher effect than LA.
Using ELISA, administration of LA or SSO for 8 weeks showed significant induction of BDNF serum levels, in comparison to the vehicle (****P<0.0001 vs. *P<0.05, respectively; Fig. 5). Indeed, the concentration of BDNF increased from 128.7±7.48 µ mol/ml in vehicle mice to 163.8±8.12 in LA-treated mice and 209.4±6.31 µ mol/ml in SSO-treated mice. It is worth noting that the increase in BDNF serum levels was significantly higher (*P<0.05) in SSO-, compared to LA-treated mice (Fig. 5).
Neurogenesis and survival of neurons in the hypothalamic nuclei are critical in food intake and other related body functions. A dramatic decline in neural stem/progenitor cell proliferation and self-renewal occurs due to aging, chronic stress, and central nervous system disorders. This may lead to weight gain and related diseases [19]. Indeed, herbal therapy is considered the first line of treatment for most types of diseases in developing countries [20]. Recently, we have shown that SSO affected NSC proliferation and differentiation in vitro [16]. Therefore, we designed the current in vivo study to translate the previous in vitro data into an in vivo mouse model.
In this study, the number of neurospheres increased significantly during the 8-week administration of SSO, which contains 56 µM of natural LA, but did not increase by synthetic LA. Moreover, not only SSO but also synthetic LA demonstrated a significant increase in the number of neurosphere-generated single cells, cultured from hypothalamic NSCs. The number of neurospheres and single cells was considerably higher in SSO, compared to LA, but did not reach statistical significance. In accordance, we already showed that SSO promotes the proliferation of NSCs in vitro [16]. In addition, it has been revealed that LA enhanced the maintenance of embryonic NSCs [21]. It is worth noting that SSO contains mainly LA (73%), in addition to 15.1% OA and 5.7% PA. Interestingly, while one study reported protective effects of OA in neurological diseases [22], two other studies showed deleterious effects of OA and PA in neurological diseases; respectively [23, 24].
On the other hand, immunofluorescent staining of the hypothalamus showed that astrocytes are not affected by SSO or LA. However, both caused a significant upregulation in the number of mature and immature neurons (NeuN+ and DCX+ cells), with a significantly stronger effect for SSO in increasing the number of neurons, in comparison to LA. This observation was by the results of our previous in vitro study [16]. Furthermore, Okui et al. [25], reported that conjugated LA which is an isomer of LA, but not LA, increases the neuronal differentiation of embryonic NSCs.
Altogether, we found that an enhancement in the proliferation rate of SSO-treated mice was coincident with an increase in the differentiation activity of hypothalamic cells toward neurons. This simultaneous increase was already observed in vitro [16], where SSO promoted the proliferation and stemness activity of NSCs via the Notch 1 signaling pathway. Indeed, when seeded in differentiating media, cells differentiated to all three neural lineages (astrocytes, oligodendrocytes, and neurons), of which only the neuronal differentiation was statistically significant, in comparison to controls. This simultaneous increase suggests an overall increase in cell viability or proliferation rate in the presence of SSO.
It has been reported that the proportion of newborn neurons among newborn cells in the adult rodent hypothalamus is considerably lower than that of the SGZ and SVZ regions (1%–37% vs. 70%–100%) [6, 26]. However, hypothalamic neurogenesis can be stimulated by intrinsic factors [27] including fibroblast growth factor 2 [7], insulin-like growth factor [28], and BNDF [29]. Particularly, BDNF has a high potential to transit the blood-brain barrier (BBB) in both directions [30] which makes it an important factor in hypothalamic neurogenesis. Moreover, it has been suggested that serum levels of BDNF represent an important reserve pool for the brain [31]. Therefore, BDNF serum levels were measured in this study and were found to be significantly increased following the administration of SSO or LA. It is important to note that the effect of SSO on BDNF serum levels was significantly more potent than that of LA. Given the potential of BDNF to transit the BBB, serum BDNF could be an indicator of its brain levels. In accordance, it has been demonstrated that infusion of BDNF into the lateral ventricle of the adult rat caused the generation of new neurons in the hypothalamus [9]. Although we have determined the population of newly produced neurons through co-labeling with BrdU and neuronal markers, three phenomena were increased concurrently; (1) the rate of neural proliferation, (2) the number of immature (DCX+ cells) and mature neurons (NeuN+ cells), and (3) BDNF serum levels. Taken together, this suggests that overall increases in hypothalamic cell proliferation and the stimulatory effects of BDNF caused the induction of neural cell differentiation toward neurons. Importantly, DCX-positive cells resembling immature and developing neurons have been recently confirmed to occur in the hypothalamus [32].
DCX is expressed by NPCs and immature neurons. NPCs begin to express DCX while actively dividing, and their neuronal daughter cells continue to express DCX for 2 to 3 weeks, as the cells mature into neurons [33]. Down regulation of DCX begins after 2 weeks while the cells start to express NeuN, a marker for mature neurons. Due to the nearly exclusive expression of DCX in developing neurons, this protein has been widely used as a marker for neurogenesis [34, 35]. In accordance, the increase in DCX-expressing cells in the current study suggests an increase in neurogenesis. Moreover, we hypothesize that higher BDNF serum levels following SSO administration could be associated with a higher rate of neurogenesis. In support, a study showed that a higher level of BDNF was associated with a slower rate of cognitive decline in Alzheimer's disease patients [31], which could be due to stimulation of neurogenesis by BDNF. This further supports that increasing adult neurogenesis can combat neurodegenerative diseases and cognitive decline [11].
Previous studies in rodents showed that consumption of a high-fat diet leads to endoplasmic reticulum stress [36] and apoptosis of hypothalamic neurons [37]. However, this study demonstrates that administration of SSO, a rich source of LA with OA, PA, and other fatty acids, not only induced apoptosis in hypothalamic cells but also increased neural proliferation and the population of neuronal cells, associated with an increase in BDNF serum levels.
This in vivo study is complementary to our previous in vitro work and provides further confirmation of the beneficial role of SSO on neurogenesis in the adult hypothalamus. Considering the difference between natural SSO and synthetic LA, we highlighted that administration of LA alongside other fatty acids can increase its efficiency in stimulating neurogenesis. Further investigations using various ratios of different fatty acids, particularly OA and PA, are still needed.
Acknowledgements
This work was supported by a grant from the Cell and Molecular research center of Yasuj University of Medical Sciences, Yasuj, Iran.
Notes
References
1. Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. 2004; Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 44:399–421. DOI: 10.1146/annurev.pharmtox.44.101802.121631. PMID: 14744252.

2. Ming GL, Song H. 2005; Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 28:223–50. DOI: 10.1146/annurev.neuro.28.051804.101459. PMID: 16022595.

3. Palmer TD, Ray J, Gage FH. 1995; FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 6:474–86. DOI: 10.1006/mcne.1995.1035. PMID: 8581317.

4. Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. 2008; PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 11:1392–401. DOI: 10.1038/nn.2220. PMID: 18849983. PMCID: PMC3842596.

5. Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. 2003; Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 9:439–47. DOI: 10.1038/nm837. PMID: 12627226.

6. Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. 2010; Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 32:2042–52. DOI: 10.1111/j.1460-9568.2010.07521.x. PMID: 21143659.

7. Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. 2013; α-tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 4:2049. DOI: 10.1038/ncomms3049. PMID: 23804023.

8. Eghlidospour M, Ghanbari A, Mortazavi SMJ, Azari H. 2017; Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem cells. Anat Cell Biol. 50:115–23. DOI: 10.5115/acb.2017.50.2.115. PMID: 28713615. PMCID: PMC5509895.

9. Pierce AA, Xu AW. 2010; De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 30:723–30. DOI: 10.1523/JNEUROSCI.2479-09.2010. PMID: 20071537. PMCID: PMC3080014.
10. Abbasnia K, Ghanbari A, Abedian M, Ghanbari A, Sharififar S, Azari H. 2015; The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 48:104–13. DOI: 10.5115/acb.2015.48.2.104. PMID: 26140221. PMCID: PMC4488638.

11. Poulose SM, Miller MG, Scott T, Shukitt-Hale B. 2017; Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr. 8:804–11. DOI: 10.3945/an.117.016261. PMID: 29141966. PMCID: PMC5683005.

12. Gordon N. 1997; Nutrition and cognitive function. Brain Dev. 19:165–70. DOI: 10.1016/S0387-7604(96)00560-8. PMID: 9134186.

13. Hamosh M, Salem N Jr. 1998; Long-chain polyunsaturated fatty acids. Biol Neonate. 74:106–20. DOI: 10.1159/000014017. PMID: 9691153.

14. Kelly GJ. 1984; Formation and fates of plant polyunsaturated fatty acids. Trends Biochem Sci. 9:502–3. DOI: 10.1016/0968-0004(84)90269-X.

15. Barnes PM, Bloom B, Nahin RL. 2008; Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. (12):1–23. DOI: 10.1037/e623942009-001. PMID: 19361005.

16. Ghareghani M, Zibara K, Azari H, Hejr H, Sadri F, Jannesar R, Ghalamfarsa G, Delaviz H, Nouri E, Ghanbari A. 2017; Safflower seed oil, containing oleic acid and palmitic acid, enhances the stemness of cultured embryonic neural stem cells through notch1 and induces neuronal differentiation. Front Neurosci. 11:446. DOI: 10.3389/fnins.2017.00446. PMID: 28824367. PMCID: PMC5540893. PMID: 66efc589a6fa45e19842f4d4ae55e563.

17. Sabzalian MR, Saeidi G, Mirlohi A. 2008; Oil content and fatty acid composition in seeds of three safflower species. J Am Oil Chem Soc. 85:717–21. DOI: 10.1007/s11746-008-1254-6.

18. Li J, Tang Y, Cai D. 2012; IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 14:999–1012. DOI: 10.1038/ncb2562. PMID: 22940906. PMCID: PMC3463771.

19. Calapai G. 2008; European legislation on herbal medicines: a look into the future. Drug Saf. 31:428–31. DOI: 10.2165/00002018-200831050-00009. PMID: 18422385.
20. Kamelia E, Miko H, Karo MB, Hatta M. 2016; Neurogenesis and brain-derived neurotrophic factor levels in herbal therapy. Int J Res Med Sci. 4:4654–8. DOI: 10.18203/2320-6012.ijrms20163757.

21. Sakayori N, Osumi N. 2013; Polyunsaturated fatty acids and their metabolites in neural development and implications for psychiatric disorders. Curr Psychopharmacol. 2:73–83. DOI: 10.2174/2211556011302010073.

22. Park YS, Jang HJ, Lee KH, Hahn TR, Paik YS. 2006; Prolyl endopeptidase inhibitory activity of unsaturated fatty acids. J Agric Food Chem. 54:1238–42. DOI: 10.1021/jf052521h. PMID: 16478242.

23. Kim YJ, Nakatomi R, Akagi T, Hashikawa T, Takahashi R. 2005; Unsaturated fatty acids induce cytotoxic aggregate formation of amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutants. J Biol Chem. 280:21515–21. DOI: 10.1074/jbc.M502230200. PMID: 15799963.

24. Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. 2008; Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 14:133–45. DOI: 10.3233/JAD-2008-14202. PMID: 18560126. PMCID: PMC2670571.

25. Okui T, Hashimoto M, Katakura M, Shido O. 2011; Cis-9,trans-11-conjugated linoleic acid promotes neuronal differentiation through regulation of Hes6 mRNA and cell cycle in cultured neural stem cells. Prostaglandins Leukot Essent Fatty Acids. 85:163–9. DOI: 10.1016/j.plefa.2011.06.001. PMID: 21723718.

26. Lledo PM, Alonso M, Grubb MS. 2006; Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 7:179–93. DOI: 10.1038/nrn1867. PMID: 16495940.

27. Recabal A, Caprile T, García-Robles MLA. 2017; Hypothalamic neurogenesis as an adaptive metabolic mechanism. Front Neurosci. 11:190. DOI: 10.3389/fnins.2017.00190. PMID: 28424582. PMCID: PMC5380718.

28. Pérez-Martín M, Cifuentes M, Grondona JM, López-Avalos MD, Gómez-Pinedo U, García-Verdugo JM, Fernández-Llebrez P. 2010; IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 31:1533–48. DOI: 10.1111/j.1460-9568.2010.07220.x. PMID: 20525067.

29. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. 2001; Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 21:6706–17. DOI: 10.1523/JNEUROSCI.21-17-06706.2001. PMID: 11517260. PMCID: PMC6763082.

30. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. 1998; Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 37:1553–61. DOI: 10.1016/S0028-3908(98)00141-5. PMID: 9886678.

31. Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. 2011; Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 14:399–404. DOI: 10.1017/S1461145710001008. PMID: 20860877.

32. Batailler M, Droguerre M, Baroncini M, Fontaine C, Prevot V, Migaud M. 2014; DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: a comparative study between mouse, sheep, and human tissues. J Comp Neurol. 522:1966–85. DOI: 10.1002/cne.23514. PMID: 24288185.

33. Ballout N, Frappé I, Péron S, Jaber M, Zibara K, Gaillard A. 2016; Development and maturation of embryonic cortical neurons grafted into the damaged adult motor cortex. Front Neural Circuits. 10:55. DOI: 10.3389/fncir.2016.00055. PMID: 27536221. PMCID: PMC4971105. PMID: 1e4b5c3689444f069c3ac3b07878ec42.

34. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. 2003; Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10. DOI: 10.1002/cne.10874. PMID: 14574675.

35. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. 2005; Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 21:1–14. DOI: 10.1111/j.1460-9568.2004.03813.x. PMID: 15654838.

36. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG Jr, Ozcan U. 2009; Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9:35–51. DOI: 10.1016/j.cmet.2008.12.004. PMID: 19117545.

37. Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. 2009; High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 4:e5045. DOI: 10.1371/journal.pone.0005045. PMID: 19340313. PMCID: PMC2661137. PMID: a7d0e63a092644f5bc26ec40e48366f8.

Fig. 1
The effect of SSO and LA on NSC neurosphere formation. (A) Representative images of neurospheres in the different groups. Scale bar=100 μm. (B) SSO, but not LA, significantly increased neurosphere formation. (C) Cell counts obtained from neurospheres showed an increase of the mean cell number for both SSO or LA, compared to vehicle. Data were expressed as mean±standard error of the mean and each experiment included 10 replicates per condition (n=10). Statistical analyses were performed by one-way analysis of variance followed by Tukey’s test. Significance is indicated by *P<0.05, **P<0.01, and ***P<0.001. SSO, safflower seed oil; LA, linoleic acid; NSC, neural stem cell; Ctrl, control mice; Vehicle, vehicle mice.
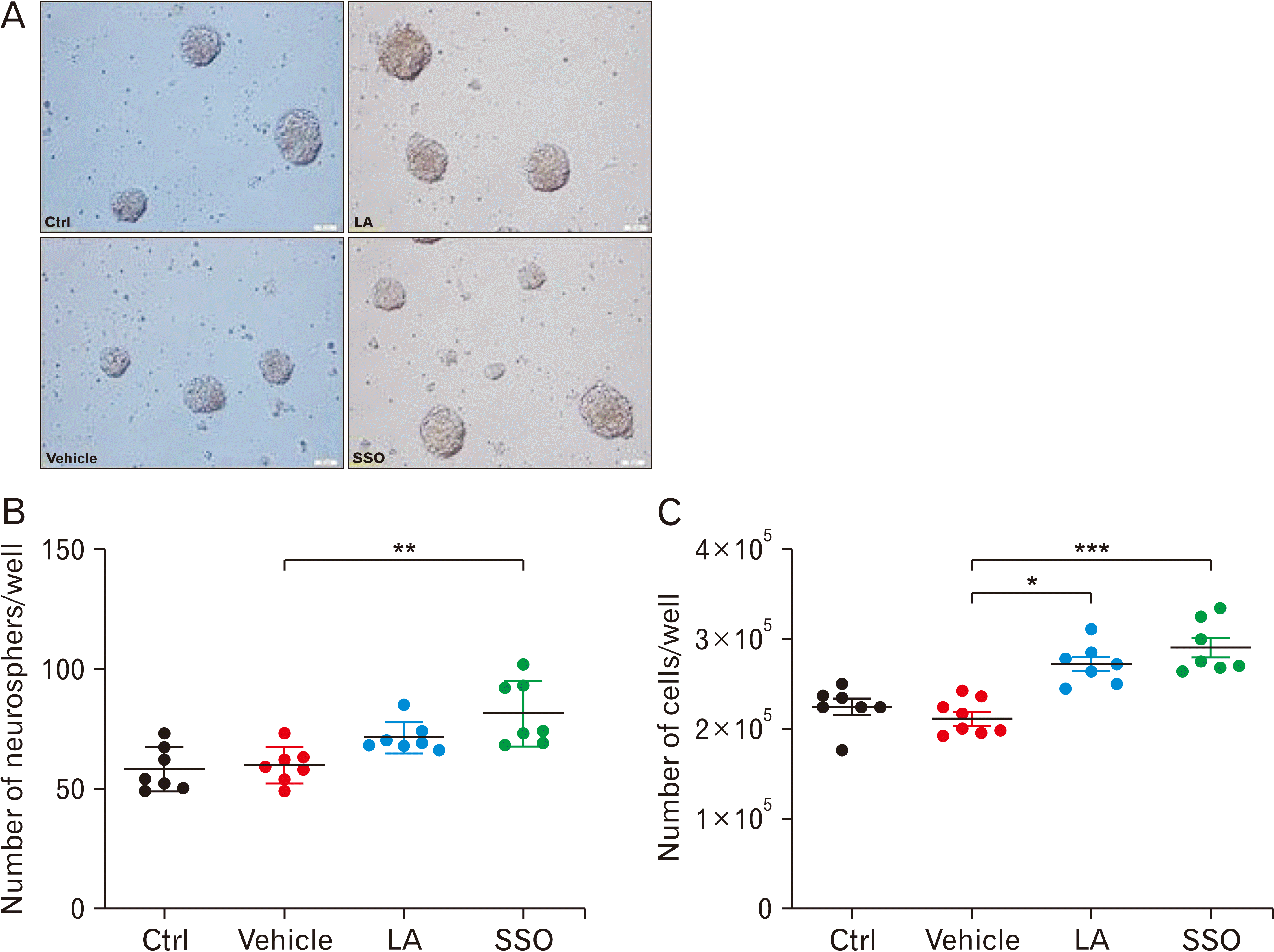
Fig. 2
Immunofluorescence staining of hypothalamic astrocytes in vivo. (A) Astrocytes were labeled by an antibody against GFAP. Scale bar=100 μm. (B) Quantitative data of astrocytes in the hypothalamus in vivo. Data are presented as mean±standard error of the mean. Statistical analyses were performed by one-way analysis of variance followed by Tukey’s test. GFAP, glial fibrillary acidic protein; Ctrl, control mice; Vehicle, vehicle mice; LA, linoleic acid; SSO, safflower seed oil.

Fig. 3
Immunofluorescence staining of hypothalamic mature neurons in vivo. (A) Mature neurons were labeled by an antibody against NeuN. Scale bar=100 μm. (B) Quantitative data of neurons in the hypothalamus in vivo. Data are presented as mean±standard error of the mean. Statistical analyses were performed by one-way analysis of variance followed by Tukey’s test. Significance is indicated by *P<0.05 and ****P<0.0001. NeuN, neuronal nuclei; Ctrl, control mice; Vehicle, vehicle mice; LA, linoleic acid; SSO, safflower seed oil.

Fig. 4
Immunofluorescence staining of hypothalamic immature neurons in vivo. (A) Immature neurons were labeled by an antibody against DCX. Scale bar=200 μm. (B) Quantitative data of immature neurons in the hypothalamus in vivo. Data are presented as mean±standard error of the mean. Statistical analyses were performed by one-way analysis of variance followed by Tukey’s test. Significance is indicated by *P<0.05, ***P<0.01, and ****P<0.0001. DCX, doublecortin; Ctrl, control mice; Vehicle, vehicle mice; LA, linoleic acid; SSO, safflower seed oil.
Fig. 5
Serum levels of BDNF. (A-D) A scatter plot with correlation coefficients for SSO and LA groups. Seven mice per group (n=7) were used. Statistical analyses were performed by one-way analysis of variance followed by Tukey’s test. Significance is indicated by *P<0.05, and ****P<0.0001. BDNF, brain-derived neurotrophic factor; NeuN, neuronal nuclei; DCX, doublecortin; Ctrl, control mice; Vehicle, vehicle mice; LA, linoleic acid; SSO, safflower seed oil.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download