This article has been
cited by other articles in ScienceCentral.
Abstract
Although in counterpart, the sphenopalatine artery (SPA), has been well described in the medical literature, the sphenopalatine vein (SPV) has received scant attention. Therefore, the present anatomical study was performed. Additionally, we discuss the variations, embryology, and clinical significance of the SPV. Adult cadaveric specimens underwent dissection of the SPV. In addition, some specimens were submitted for histological analysis of this structure. The SPV was found to drain from the sphenoidal sinus and nasal septum. Small tributaries traveled through the nasal septum with the posterior septal branches of the SPA and nasopalatine nerve. The SPA and SPV were found to travel through the sphenopalatine foramen and another tributary was found to perforate the medial plate of the pterygoid process and to connect to the pterygoid venous plexus which traveled lateral to the medial plate of the pterygoid process. The vein traveled through the posterior part of the lateral wall of the nasal cavity with the posterior lateral nasal branches of the SPA and the lateral superior posterior nasal branches of the maxillary nerve. To our knowledge, this is the first anatomical study on the SPV in humans. Data on the SPV provides an improved anatomical understanding of the vascular network of the nasal cavity. Developing a more complete picture of the nasal cavity and its venous supply might help surgeons and clinicians better manage clinical entities such as posterior epistaxis, cavernous sinus infections, and perform endoscopic surgery with fewer complications.
Go to :

Keywords: Arteries, Cadaver, Veins, Anatomy, Nose
Introduction
In human anatomy, the sphenopalatine vein (SPV) has not been well characterized or studied in and it is typically, simply described as arising from the sphenopalatine foramen along with the sphenopalatine artery (SPA) and nasopalatine nerve. However, its location in the posterior nasal cavity implicates the vein in a variety of clinical pathologies which make knowledge of the anatomy critical for understanding various diagnoses and surgical procedures in this area,
e.g., epistaxis and posterior nasal neurectomy, cavernous sinus infection, and nasal congestion in rhinitis. Although vascular network called “Woodruff’s plexus” has been well discussed, there is a lack of evidence of the vascular system in the area [
1]. Therefore, the present anatomical study was performed to better elucidate this structure of the nasal cavity.
Go to :

Materials and Methods
Eight embalmed cadaveric heads, derived from four males and four females, were used in this study. The mean age at the time of death was 85 years (range 78–94 years). Three were latex injected specimens and were used for gross anatomical dissection using the surgical microscope (Zeiss). Five specimens were used for histological observation.
Gross anatomy dissection
Three cadaveric heads were bisected using a bone saw and separated into two parts, one with the nasal septum and the other without (
Fig. 1). The SPV and its tributaries were dissected carefully and its relationship with the SPA, nasopalatine nerve, and sphenopalatine foramen was documented.
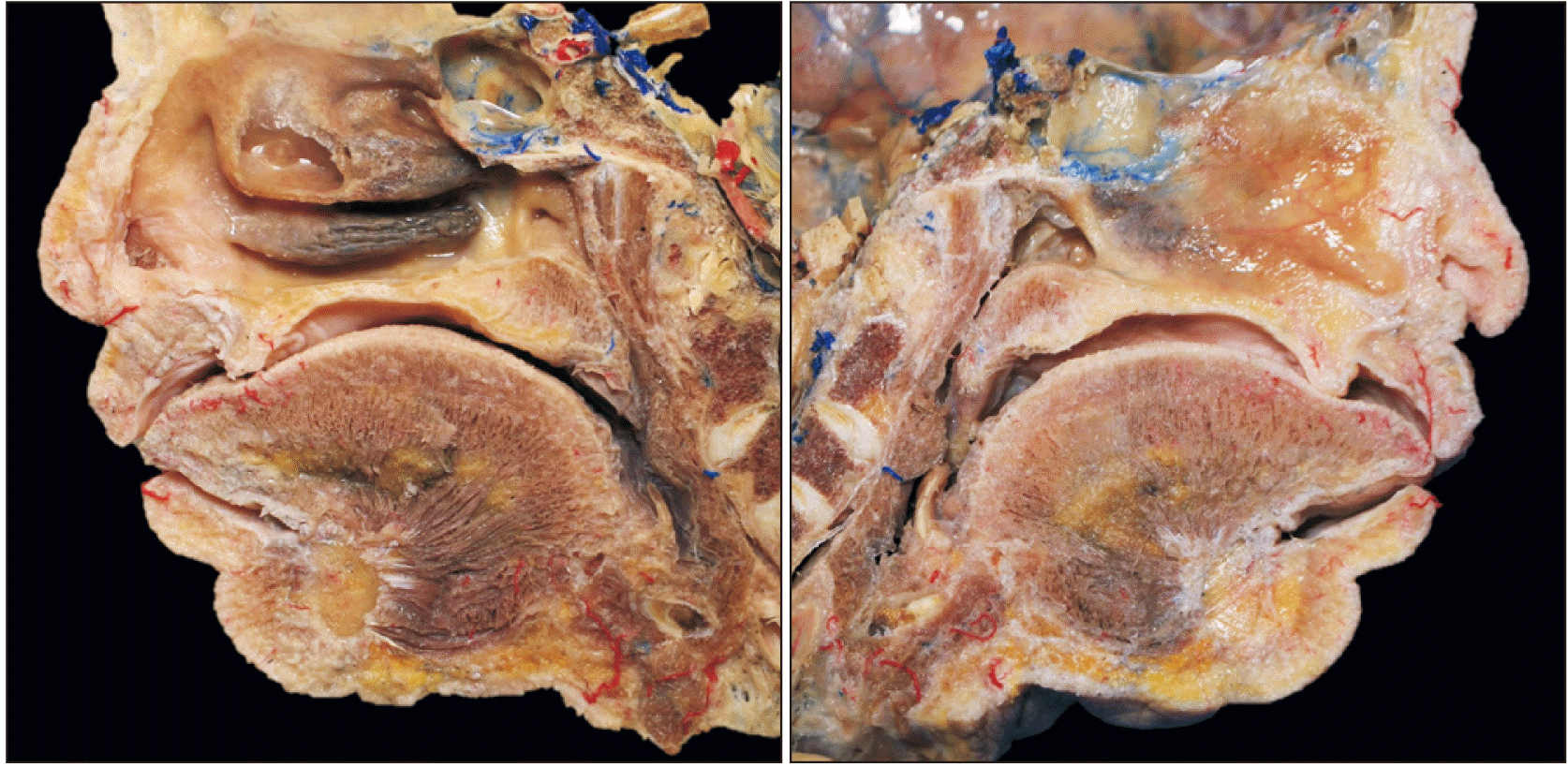 | Fig. 1Cadaveric head bisected using a bone saw and separated into two parts, one with the nasal septum (the right image) and the other without (the left image). 
|
Histological observation
Five non-injected cadaveric heads were used for microscopic observation. The nasal septum and adjacent palate were harvested en bloc and an additional coronal section was made for histologic examination in three specimens (
Fig. 2). In other two specimens, the lateral nasal wall with the nasal septum was harvested for histology.
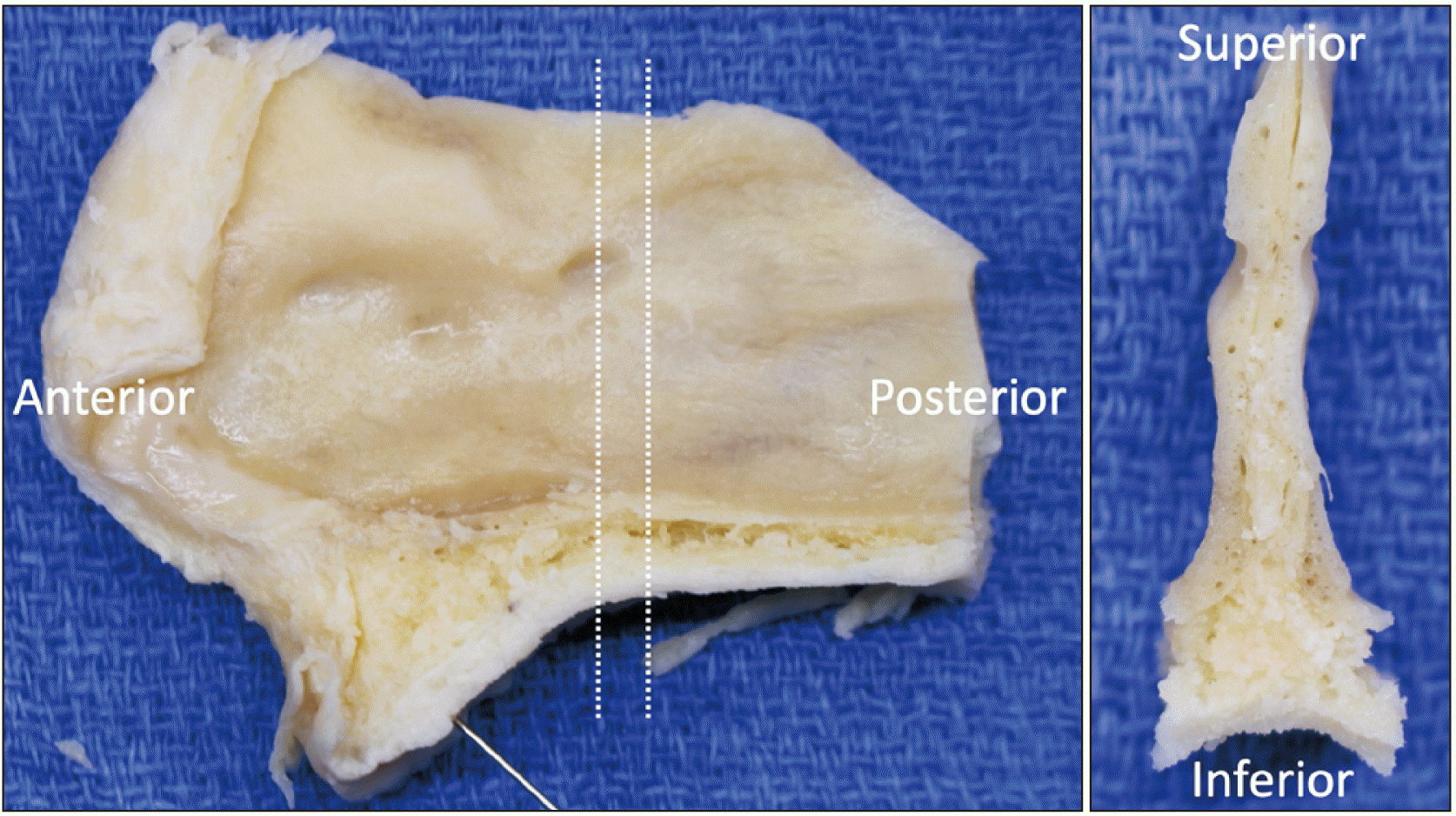 | Fig. 2The nasal septum and adjacent palate harvested from the midline (the left image). The additional coronal section was made for histological analysis (the right image). 
|
All of five specimens were decalcified for 14 days using hydrochlonic acid and embedded in paraffin. Then, 5 µm slices were cut with a microtome. All five specimens were stained with Masson-trichrome.
No previous surgical scars were observed in the dissected area of any specimens. The present study was performed in accordance with the requirements of the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013). The authors state that every effort was made to follow all local and international ethical guidelines and laws that pertain to the use of human cadaveric donors in anatomical research [
2].
Go to :

Results
Gross anatomy dissection
Nasal septum
The SPV and related venous plexus were observed at the inferior aspect of the sphenoidal sinus (
Fig. 3). Following removal of the mucosa of the nasal septum, the SPV was found to drain from the sphenoidal sinus and nasal septum. Small tributaries traveled through the nasal septum with the posterior septal branches of the SPA and nasopalatine nerve. Here, we call these venous tributaries “posterior septal branches of the SPV.” The findings in the three specimens were consistent.
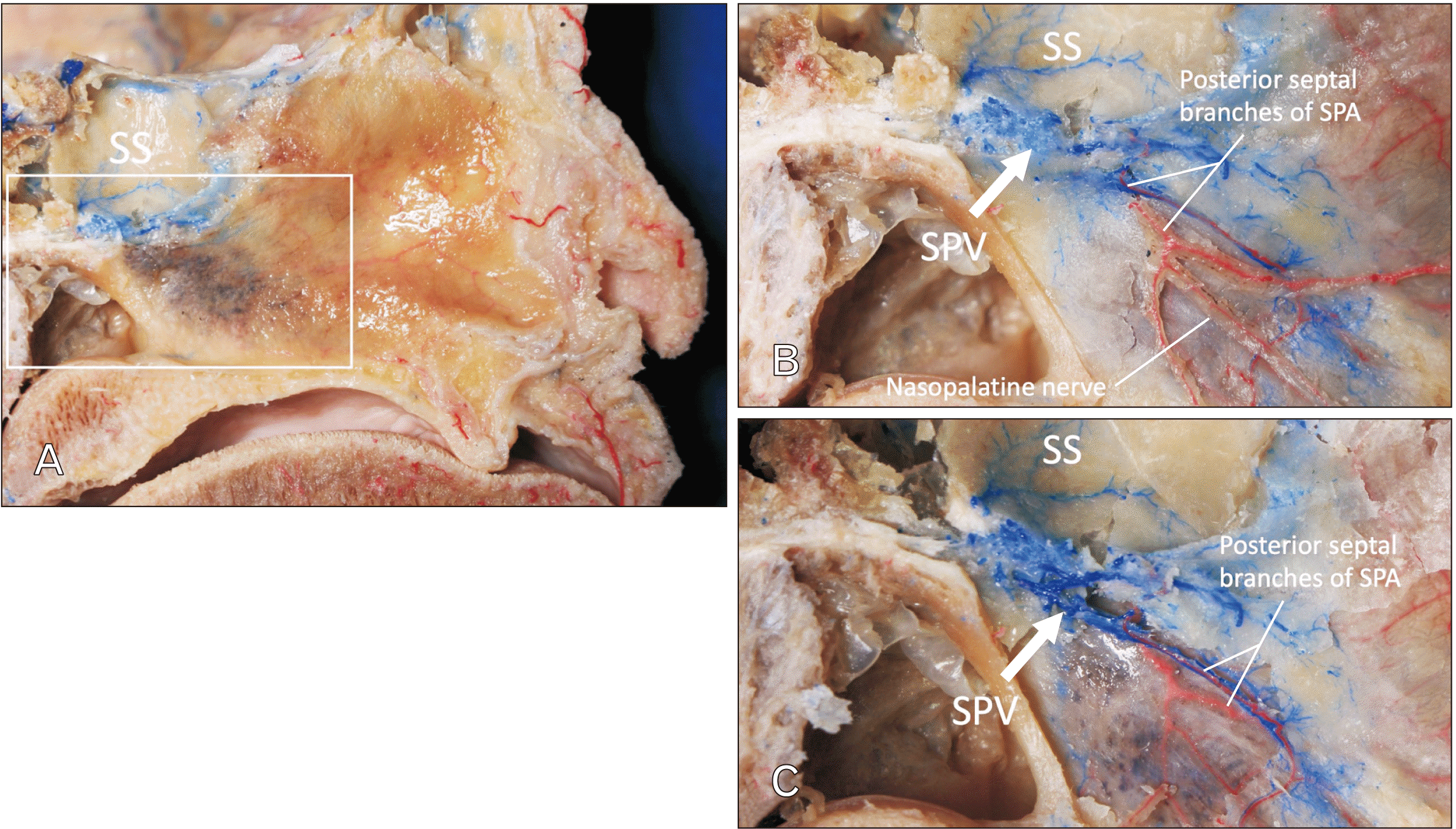 | Fig. 3Posterior part of the nasal septum (midsagittally hemisected head). (A) Specimen prior to dissection. (B) Following removal of the mucosa of the right side of the nasal septum. SPV drains from the SS and nasal septum. (C) Following careful bony removal around the SPV, note that the SPV courses with the posterior septal branches of the SPA. SS, sphenoidal sinus; SPV, sphenopalatine vein; SPA, sphenopalatine artery. 
|
Posterior part of the lateral wall of the nasal cavity
Following removal of the mucosa of the posterior part of the lateral wall of the nasal cavity, the SPA and SPV were found to travel through the sphenopalatine foramen and another tributary was found to perforate the medial plate of the pterygoid process and to connect to the pterygoid venous plexus which traveled lateral to the medial plate of the pterygoid process. This vein also drained from the sphenoidal sinus (
Fig. 4). The vein traveled through the posterior part of the lateral wall of the nasal cavity with the posterior lateral nasal branches of the SPA and the lateral superior posterior nasal branches of the maxillary nerve. Here, we call these venous tributaries “posterior lateral nasal branches of the SPV.” The findings among the three specimens were consistent.
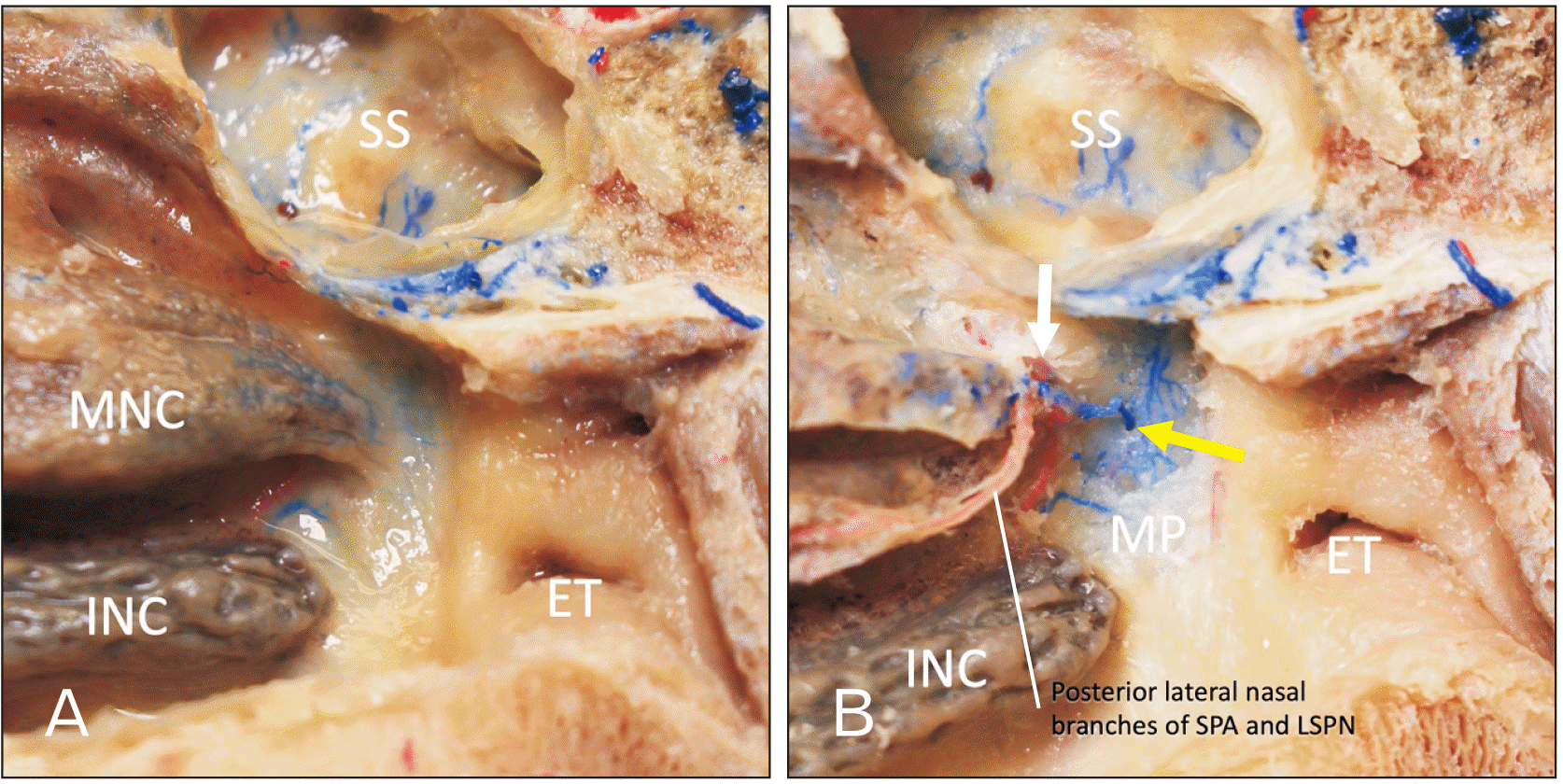 | Fig. 4Posterior part of the lateral wall of the nasal cavity. (A) Specimen prior to dissection. (B) Following removal of the mucosa. Note the SPA and vein go through the sphenopalatine foramen (white arrow) and another tributary (yellow arrow) perforates the MP to connect with the SPV which runs with the posterior lateral nasal branches of SPA. ET, eustachian tube; INC, inferior nasal concha; MNC, middle nasal concha; MP, medial plate of pterygoid process; LSPN, lateral superior posterior nasal branches of the maxillary nerve; SS, sphenoidal sinus; SPV, sphenopalatine vein; SPA, sphenopalatine artery. 
|
Histological observations
Nasal septum
Multiple posterior septal branches of the SPA and SPV are seen in close proximity to one another (
Fig. 5) and the findings among the three specimens were consistent.
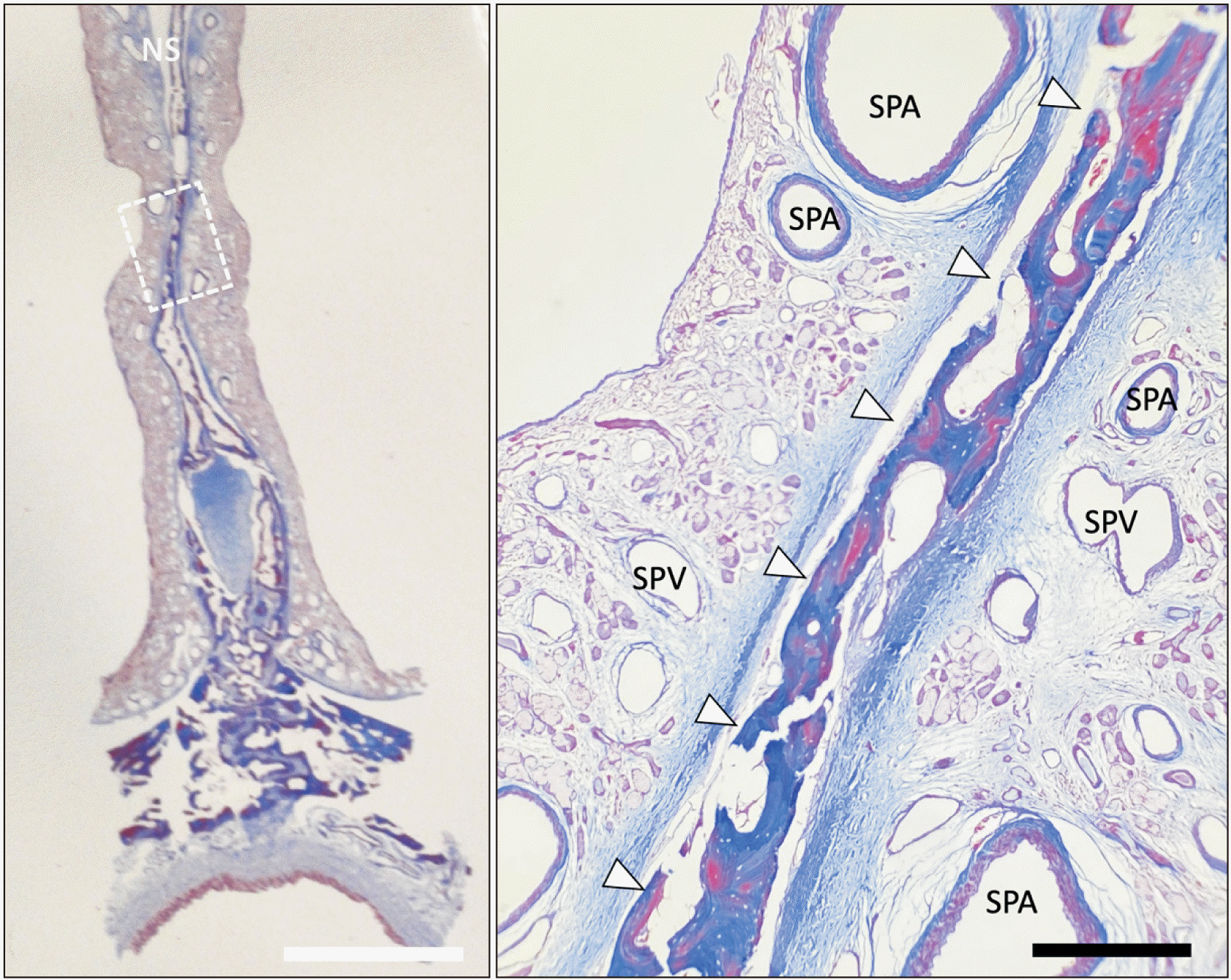 | Fig. 5Masson-trichrome stain of the NS. The right image is a magnified image of dotted rectangular area of the left one. Note the posterior septal branches of the SPA and SPV are shown with close proximity. The perpendicular plate of the ethmoid bone is shown with arrowheads. NS, nasal septum; SPV, sphenopalatine vein; SPA, sphenopalatine artery. White scale bar: 5 mm, black scale bar: 500 μm. 
|
Nasal septum and the lateral wall of the nasal cavity
Multiple posterior lateral nasal branches of the SPA and SPV are shown in close proximity (
Fig. 6) and these findings in the two specimens were consistent.
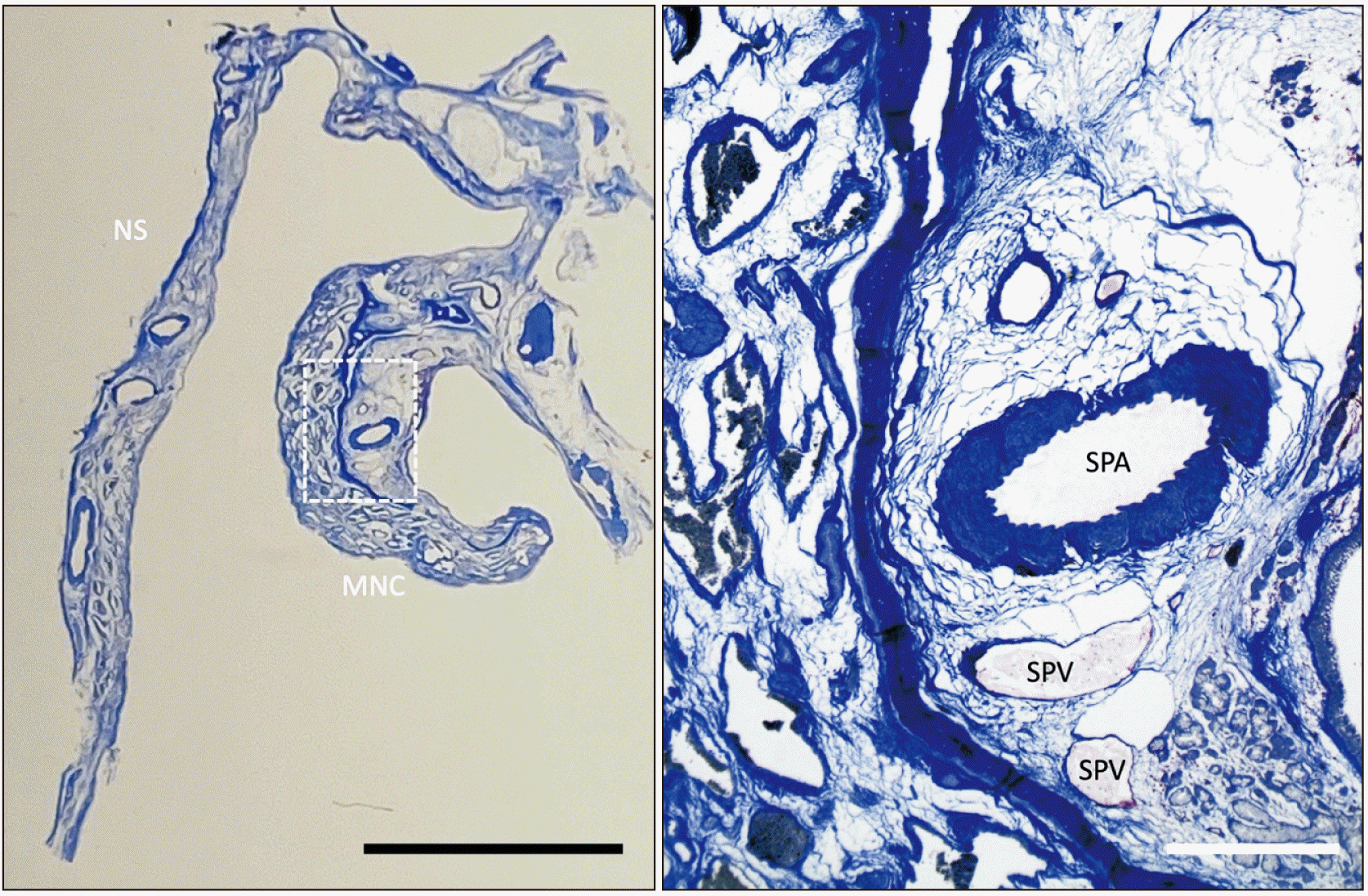 | Fig. 6Masson-trichrome stain of the NS and MNC. The right image is a magnified image of dotted rectangular area of the left one. Note that the posterior lateral nasal branches of the SPA and SPV are shown in close proximity. NS, nasal septum; MNC, middle nasal concha; SPV, sphenopalatine vein; SPA, sphenopalatine artery. Black scale bar: 5 mm, white scale bar: 500 μm. 
|
Go to :

Discussion
Anatomy
The venous network in the nasal cavity is less described in the literature compared to the arterial vasculature. Descriptions on the venous drainage of the nasal cavity are limited to simple statements such as that they ‘accompany the arteries’ and form a rich submucosal network of plexuses in the posterior region of the septum and middle and inferior conchae. The nasal venous network can be divided further by large veins at their anterior and posterior extremities. Veins in the anterior nasal cavity communicate with the greater palatine veins, which eventually drain into the ophthalmic or facial veins. Veins in the posterior nasal cavity drain into the ethmoidal veins, which pass through the cribriform plate to the superior sagittal sinus, and also into the SPV, which join the venous plexuses of the palate and nasopharynx. The SPV receives drainage from vessels of the posterior nasal cavity and passes through the sphenopalatine foramen to join the pterygoid plexus in the infratemporal fossa [
1,
3]. The SPV can penetrate the medial plate of the pterygoid process to join the pterygoid venous plexus (
Fig. 4). It might also drain from the medial wall of the maxillary sinus [
4].
Embryology
The embryological origins of the SPV have not been described in the literature but examination of the development of the SPA and related nasal venous structures may provide insight into its embryology. It is understood that the aortic arch branches to the first pharyngeal arch form around the fourth to the fifth week of embryologic development. The SPA, including other branches of the maxillary artery are formed from the aortic sac via the aortic arch [
5]. The SPV might develop in the similar period of time.
Clinical significance
Epistaxis and posterior nasal neurectomy
The nasal cavity is richly vascularized with a high pressure blood supply from both the internal and external carotid arteries [
1]. Epistaxis represents one of the most common ear, nose and throat conditions encountered by emergency providers in the United States [
6]. Posterior epistaxis occurs in approximately 10%–20% of cases and is attributable to bleeding that arises from branches of the sphenopalatine vessels. It is widely recognized that posterior nasal cavity bleeding is more profuse and harder to control than anterior bleeding [
5-
7]. An understanding of the SPV and its relationship to the nasal angioarchitecture may prove useful when performing endoscopic procedures such as ligation for control of posterior epistaxis and posterior nasal neurectomy.
Cavernous sinus infection
The nasal venous system communicates with the ophthalmic and facial veins that drain into the cavernous sinus. The rich valveless anastomotic network, including the SPV, may make it more likely for infection in the region to spread to the cavernous sinus with the potential for thrombosis [
3,
8].
Nasal congestion in rhinitis
The posterior lateral nasal branches and posterior septal branches of the SPV are the only venous pathways in the posterior nasal cavity as shown in the present study. As Lung speculated, both sympathetic and parasympathetic fibers are involved in causing nasal congestion in rhinitis [
9]. Whether the autonomic stimulation increases or decreases the venous outflow, the SPV can be the pathway of drainage from the posterior nasal cavity, inferior aspect of the sphenoidal sinus, and medial wall of the maxillary sinus. In cats, stimulation of the cervical sympathetic trunk decreases both nasal airway resistance and sphenopalatine venous outflow [
10,
11]. On the other hand, stimulation of the vidian nerve (pterygopalatine ganglion) [
12] can increase both nasal airway resistance and sphenopalatine venous outflow [
11,
13]. In dogs, it is known that blood from the nasal mucosa drains via two main pathways
i.e., the anterior two thirds via the external (dorsal) nasal vein and posterior one third via the SPV [
14]. Lung [
9] speculated that both sympathetic and parasympathetic fibers are involved in causing nasal congestion in rhinitis.
In conclusion, to our knowledge, this is the first anatomical study on the SPV in humans. Data on the SPV provides an improved anatomical understanding of the vascular network of the nasal cavity. The localization of the SPV near important posterior nasal landmarks such as the SPA and sphenopalatine foramen should also warrant greater clinical considerations and more accurate anatomic descriptions of the veins in the nasal cavity. Developing a more complete picture of the nasal cavity and its venous supply might help surgeons and clinicians better manage clinical entities such as posterior epistaxis, cavernous sinus infections, and perform endoscopic surgery with fewer complications.
Go to :









 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download