Abstract
An oroantral fistula (OAF) or oroantral communication (OAC) is an opening between the oral cavity and the maxillary sinus. If left untreated, these openings may cause chronic maxillary sinusitis. Although small defects (diameter <5 mm) may close spontaneously, larger communications require surgical intervention. Various studies have been conducted on OAC closure using a platelet-rich fibrin (PRF) membrane; most of these prior studies have involved simple direct application of PRF clots. This study introduces a new “double-barrier technique” using PRF for closure of an OAF involving sinus mucosal lifting and closure. The PRF material is inserted into the prepared maxillary sinus space, and the buccal advancement flap covers the oral side. This technique was successfully used to treat two patients with chronic OAF in the posterior maxillary region after implant removal or tooth extraction. The use of a PRF membrane in a double-barrier technique may have advantages in soft-tissue healing and could enable easy closure of chronic OAF with minimal trauma.
An oroantral communication (OAC) is an opening between the oral cavity and the maxillary sinus; if left untreated, epithelialization may occur and develop into an oroantral fistula (OAF). Various factors, including lack of bone in the sinus floor and proximity between the maxillary posterior teeth and maxillary sinus, may increase the risk of OAF1. The main etiologic factor of OAC is extraction of the maxillary molars or premolars. Other possible causes of OAC include maxillary cyst, tumor removal, trauma, and dental implant surgery2-4.
Small OACs (<3 mm in diameter) without infection or sinusitis may close spontaneously5,6. However, defects >5 mm always require surgical closure7. If left untreated, 50% of patients may experience sinusitis within 48 hours, and 90% may develop sinusitis within two weeks. The fusion of the oral epithelium and the Schneiderian membrane leads to the formation of an OAF5,6. Untreated fistulas often lead to chronic OAF and are resistant to treatment. Patients with chronic OAF show generalized mucosal thickening8 and often suffer from post-nasal drainage, purulent discharge through the fistula, perception of liquid in the nostril, and nasal congestion5.
During the past few decades, several surgical techniques have been developed to treat OAF. These surgical procedures aim to remove the diseased tissue, including the thickened epithelium, and close the fistula8. Flap closures have been preferred to close an OAF; buccal advancement, palatal rotating flap, and buccal fat pad grafting are the most commonly used techniques9. Despite many advantages, these local flap closures have several drawbacks, such as postoperative pain, a prolonged healing period, a reduction in the depth of the buccal sulcus, the need for vestibuloplasty10, risk of flap failure, and an inability to repeat the same flap surgery11. To overcome these disadvantages, various alternative techniques have been reported, such as third molar transplantation12, the Bio-Oss-Bio-Gide sandwich technique13, hydroxyapatite blocks14, and AlloDerm closures11. However, these alternatives are not frequently implemented because of their high cost and lack of standardized methods5.
Since its introduction in 2006, platelet-rich fibrin (PRF) has been widely used in oral surgery. Growth factors, such as platelet-derived growth factor (PDGF) and circulating stem cells, are enmeshed within the PRF matrix to encourage optimal healing15. PRF has several advantages over other membranes. Notably, it accelerates the healing of soft and hard tissues, especially at infected sites, as this matrix contains leukocytes and promotes their migration. Various studies have been conducted on OAC and OAF closure using PRF. However, these techniques are heterogeneous, and most studies have performed a simple direct application of the PRF clot16.
This study was conducted to introduce a “double-barrier technique” using PRF for chronic OAF closure. In this procedure, an incision is made along the border of the normal and inflamed tissues, which forms from fusion of the sinus membrane and oral mucosa. Instead of being excised, the thickened sinus membrane and soft tissue tract are conserved to close the antral fistula. The sinus mucosa that contains inflamed tissue is rotated, elevated, and sutured to close the antral side as the first layer. Next, the PRF membrane is inserted into the prepared sinus space for soft tissue enhancement and inflammation control. A full-thickness buccal advancement flap is prepared and sutured over the PRF-filled fistula.
Two male patients with chronic OAF, which developed after tooth extraction (Patient No. 1, aged 47 years) and dental implant removal (Patient No. 2, aged 48 years), were treated with the double-barrier technique using PRF at the Department of Oral and Maxillofacial Surgery at Kyung Hee University Hospital at Gangdong (Seoul, South Korea). Both patients were free of systemic disease and were non-smokers and non-drinkers. Their fistulas were left untreated for more than four months. History collection and clinical examination were performed to determine the patients’ signs and symptoms, the period of fistula opening, and previous surgical interventions. The Valsalva maneuver was used to diagnose OAF. Radiographic examinations, including panoramic radiography and cone-beam computed tomography (CBCT) (CS9600; Carestream Dental), were conducted to evaluate maxillary sinusitis and defects of the fistula site.
Under local anesthesia using a 2% lidocaine with 1:100,000 epinephrine injection, a circumferential incision was made 1 to 2 mm around the border of the OAF. An additional crestal incision was made on the mesial and distal borders of the OAF and extended to a vertical incision to form a buccal advancement flap. The margin of the circumferential incision was determined along the inflamed mucosal lining of the oral side. As an antral flap, full-thickness sinus mucosa, including the inflamed fistulous tract, was elevated from the bony defect with a periosteal elevator and rotated into the maxillary sinus to maintain continuity with the residual sinus membrane. Subsequent sinus elevation was conducted with a curved sinus elevator, and tension-free sutures were applied using absorbable suture material (4-0 Vicryl; Ethicon) to create a closed antral flap as the first layer of the double barrier. To prepare the PRF membranes, a 20-mL sample of the patient’s blood was collected into a glass-coated plastic tube without anticoagulant and centrifuged (VS-360; Vision Scientific Co.) for 15 minutes at 3,000 rpm. Two PRF clots were inserted into the prepared sinus space. Finally, the full-thickness buccal mucoperiosteal flap was elevated and advanced into the palatal side. This advancement flap was minimally released to preserve the vestibule depth and sutured using a mattress suture interrupted with nylon (4-0 blue nylon; Ailee) to close the oral side of the fistula as the second layer of the double barrier.(Fig. 1)
Antibiotic (1,000 mg cefalexin X 3/day) and analgesic (200 mg etodolac X 3/day) were prescribed for five days, and postoperative instructions, including no nose blowing and proper oral hygiene care, were assigned to both patients. Follow-up observations were performed at one and two weeks and one and three months postoperatively. A radiological evaluation was conducted using CBCT at three months after surgery.
This study followed the Declaration of Helsinki on medical protocol and ethics and was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (KHNMC 2022-03-066).
Two cases of chronic OAF that were left untreated for longer than four months were treated with a new double-barrier technique using PRF. One week after surgery, the stiches on the oral side were removed, and both patients reported no pain or discomfort after surgery. After two weeks of healing, the wound matured, and the fistulas had completely closed. Neither patient experienced wound dehiscence, maxillary sinusitis symptoms or signs, or recurrent OAF.(Fig. 2, 3)
OAF development is a common complication after extraction of maxillary molars or premolars and implant surgeries, such as implant insertion and implant fixture removal2-4. Surgical intervention should be considered if the defect is >5 mm in diameter, and several surgical techniques have been used to treat OAF, including buccal advancement, the palatal rotational flap, and the buccal fat graft. However, these techniques have been associated with closure failure9,17, as well as postoperative pain and discomfort.
Chronic OAF usually causes severe chronic inflammatory thickening of the sinus membrane, and the aim of most surgeries is to remove the thickened epithelium along the borders of the fistula8. Several reports have described closing the antral side of the OAF or OAC by suturing the sinus mucosa as a layer. In these studies, the pathologic mucosa also was removed for infection control and drainage through the fistula18.
PRF can be easily prepared by centrifuging the patient’s blood. This matrix contains accumulated cytokines, glycan chains, and glycoproteins, which assist in soft and hard tissue healing, especially at infected sites. PRF contains PDGF, transforming growth factor beta, and insulin growth factor. It also contains cytokines, including inflammatory cytokines such as interleukin (IL)-6, IL-1β, IL-1, and tumor necrosis factor alpha; and healing cytokines such as IL-4 and vascular endothelial growth factor. These components impart anti-inflammatory properties to PDGF and accelerate angiogenesis and the formation of fibroblasts and osteoblasts15.
Various studies have been conducted on OAC and OAF closure using PRF, and most of these studies have involved simple, direct application of a PRF clot. All of these various modifications of PRF closure were successful. However, there are concerns that PRF clots may migrate into the antral cavity due to negative pressure when they are directly inserted into the OAF16.
George19 introduced the triple-layered technique that uses a PRF membrane concomitantly with a buccal advancement flap and a buccal fat pad graft. In addition to the buccal advancement flap, an additional layer, such as the buccal fat pad, can protect and promote healing of the OAF. However, fistula closure using the buccal fat pad has certain disadvantages, such as sulcular depth loss and the need for vestibuloplasty10.
In this study, the antral side of the fistula was closed with the lifted sinus mucosa containing the inflammatory-thickened epithelial tissue using tension-free sutures as the first layer of the double barrier. The maxillary sinus space was prepared by sinus membrane elevation. Two PRF clots were inserted into the prepared sinus space to provide structural and functional support for soft tissue regeneration and healing. The oral side of the OAF was closed using a full-thickness buccal advancement flap to form the second layer of the double barrier. The advancement flap protects the graft material from local irritants and keeps the first layer moist, creating favorable conditions for healing. Complete closure of the OAF was observed after two weeks of healing, and there were no complications related to OAC or OAF. In addition, the amount of gingival tissue increased around the fistula area.
PRF can be considered an autogenous biomaterial as well as a membrane. Furthermore, PRF allows the incision line to be closed early and prevents bacterial infection because it contains growth factors and has anti-inflammatory properties8. Even though closure was conducted using the maxillary sinus membrane containing thickened inflammatory epithelial tissue, successful closure of the OAF was observed due to these properties. Simultaneously, because fibroblast proliferation was induced by the PRF on the oral side, dehiscence of the flap closure did not occur, even with minimal release.
In the current study, bone defect regeneration did not take place. However, Pal et al. reported that use of PRF along with a guided tissue regeneration membrane to close the OAF can result in thin bone formation within the defect20. Therefore, further study for simultaneous or secondary sinus augmentation may be needed to create bony support for potential placement of dental implants. The double-barrier technique using PRF is not only a promising method, but also a minimally invasive technique that may be used to treat OAF in conjunction with proper case selection.
In conclusion, this study introduced a new double-barrier technique using PRF for closure of chronic OAF that involved sinus mucosal lifting and closure. The PRF was inserted into the prepared maxillary sinus space, and a buccal advancement flap covered the oral side. Due to the anti-inflammatory, angiogenic, and fibroblast-activating properties of PRF, along with the local protection provided by the double barrier, complete closure of the fistula and gingival hypertrophy were observed after two weeks of healing. These results show that this double-barrier technique using PRF is not only a promising method, but also a minimally invasive approach that can be used to treat selective patients with OAF.
Notes
Authors’ Contributions
J.W.J. has conceived and drafted the manuscript. Y.J.J. performed the surgery. Y.J.J., S.o.H., E.J.L., and R.Y.K. reviewed the paper. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (KHNMC 2022-03-066). No consent to participate was obtained since the data collected was retrospective and did not include information of personal identification.
References
1. Kim MK, Han W, Kim SG. 2017; The use of the buccal fat pad flap for oral reconstruction. Maxillofac Plast Reconstr Surg. 39:5. https://doi.org/10.1186/s40902-017-0105-5. DOI: 10.1186/s40902-017-0105-5. PMID: 28286743. PMCID: PMC5325802.

2. Bilginaylar K. 2018; The use of platelet-rich fibrin for immediate closure of acute oroantral communications: an alternative approach. J Oral Maxillofac Surg. 76:278–86. https://doi.org/10.1016/j.joms.2017.07.168. DOI: 10.1016/j.joms.2017.07.168. PMID: 28859924.

3. Cheng GL, Tatakis DN. 2020; Collagen strip technique: a novel approach for ridge preservation and concomitant oroantral communication management after implant explantation. Clin Adv Periodontics. 10:135–9. https://doi.org/10.1002/cap.10092. DOI: 10.1002/cap.10092. PMID: 32065734.

4. Noel JE, Teo NW, Divi V, Nayak JV. 2016; Use of pedicled nasoseptal flap for pathologic oroantral fistula closure. J Oral Maxillofac Surg. 74:704.e1–6. https://doi.org/10.1016/j.joms.2015.11.010. DOI: 10.1016/j.joms.2015.11.010. PMID: 26704432.

5. Demetoglu U, Ocak H, Bilge S. 2018; Closure of oroantral communication with plasma-rich fibrin membrane. J Craniofac Surg. 29:e367–70. https://doi.org/10.1097/scs.0000000000004360. DOI: 10.1097/SCS.0000000000004360. PMID: 29485557.

6. Lee CYS. 2019; Closure of an oroantral communication using leucocyte-platelet rich fibrin: a novel technique using regenerative medicine. J Dent Maxillofac Surg. 2:101–6. https://doi.org/10.18314/jdms.v2i1.1540. DOI: 10.18314/jdms.v2i1.1540.

7. Mårtensson G. 1957; Operative method in fistulas to the maxillary sinus. Acta Otolaryngol. 48:253–4. https://doi.org/10.3109/00016485709124378. DOI: 10.3109/00016485709124378. PMID: 13469323.

8. Parvini P, Obreja K, Sader R, Becker J, Schwarz F, Salti L. 2018; Surgical options in oroantral fistula management: a narrative review. Int J Implant Dent. 4:40. https://doi.org/10.1186/s40729-018-0152-4. DOI: 10.1186/s40729-018-0152-4. PMID: 30588578. PMCID: PMC6306369.

9. Abuabara A, Cortez AL, Passeri LA, de Moraes M, Moreira RW. 2006; Evaluation of different treatments for oroantral/oronasal communications: experience of 112 cases. Int J Oral Maxillofac Surg. 35:155–8. https://doi.org/10.1016/j.ijom.2005.04.024. DOI: 10.1016/j.ijom.2005.04.024. PMID: 15955666.

10. Obradovic O, Todorovic L, Pesic V. 1981; Investigations of the buccal sulcus depth after the use of certain methods of oro-antral communication closure. Bull Group Int Rech Sci Stomatol Odontol. 24:209–14. PMID: 6948590.
11. Bharadwaj G. 2016; Closure of oroantral communication (OAC) with AlloDerm (regenerative tissue matrix) - a case report. Oral Surg. 9:177–9. https://doi.org/10.1111/ors.12184. DOI: 10.1111/ors.12184.

12. Kitagawa Y, Sano K, Nakamura M, Ogasawara T. 2003; Use of third molar transplantation for closure of the oroantral communication after tooth extraction: a report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 95:409–15. https://doi.org/10.1067/moe.2003.122. DOI: 10.1067/moe.2003.122. PMID: 12686925.

13. Ogunsalu C. 2005; A new surgical management for oro-antral communication: the resorbable guided tissue regeneration membrane--bone substitute sandwich technique. West Indian Med J. 54:261–3. https://doi.org/10.1590/s0043-31442005000400011. DOI: 10.1590/S0043-31442005000400011. PMID: 16312195.

14. Zide MF, Karas ND. 1992; Hydroxylapatite block closure of oroantral fistulas: report of cases. J Oral Maxillofac Surg. 50:71–5. https://doi.org/10.1016/0278-2391(92)90201-a. DOI: 10.1016/0278-2391(92)90201-A. PMID: 1309241.

15. Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. 2006; Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101:e37–44. https://doi.org/10.1016/j.tripleo.2005.07.008. DOI: 10.1016/j.tripleo.2005.07.008. PMID: 16504849.

16. Sabri H, Sarkarat F, Mortezagholi B, Aghajani D. 2022; Non-surgical management of oro-antral communication using platelet-rich fibrin: a review of the literature. Oral Surg. 15:455–64. https://doi.org/10.1111/ors.12685. DOI: 10.1111/ors.12685.

17. Anavi Y, Gal G, Silfen R, Calderon S. 2003; Palatal rotation-advancement flap for delayed repair of oroantral fistula: a retrospective evaluation of 63 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 96:527–34. https://doi.org/10.1016/s1079-2104(03)00470-0. DOI: 10.1016/S1079-2104(03)00470-0. PMID: 14600685.

18. Lee BK. 2008; One-stage operation of large oroantral fistula closure, sinus lifting, and autogenous bone grafting for dental implant installation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 105:707–13. https://doi.org/10.1016/j.tripleo.2007.09.020. DOI: 10.1016/j.tripleo.2007.09.020. PMID: 18299230.

19. George E. 2018; Triple-layered closure of an oroantral fistula: a case report. Int J Oral Maxillofac Implants. 33:e33–6. https://doi.org/10.11607/jomi.5725. DOI: 10.11607/jomi.5725. PMID: 28518182.

20. Pal S, Rao K, Sanjenbam N, Thounaojam N, Geeta R, Bagde H. 2022; A double barrier technique in surgical closure of oroantral communication. Cureus. 14:e31671. https://doi.org/10.7759/cureus.31671. DOI: 10.7759/cureus.31671. PMID: 36545175. PMCID: PMC9762534.

Fig. 1
A schematic of the incision line (A), antral flap rotation and elevation as the first barrier (B), platelet-rich fibrin (PRF) insertion into the prepared maxillary sinus space (C), a buccal advancement flap as the second barrier (D).
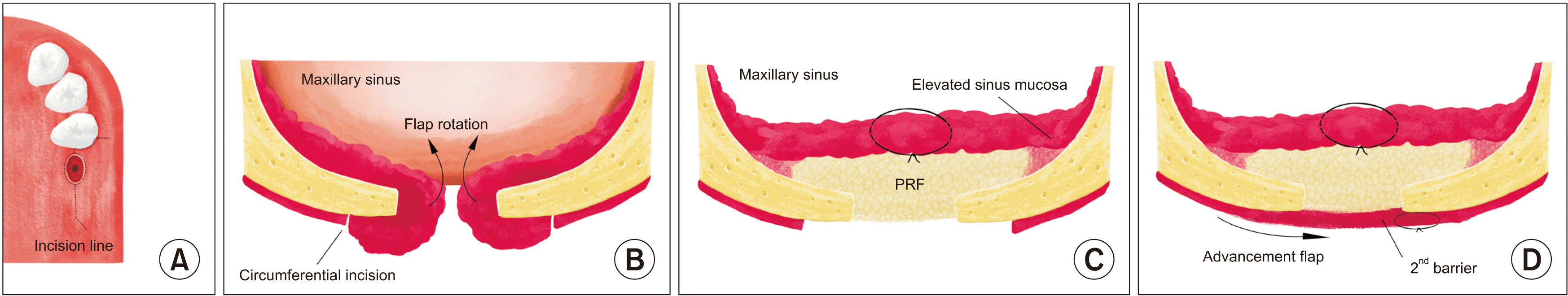
Fig. 2
A. A pre-surgery panoramic radiograph of Patient No. 1. B. The cone-beam computed tomography view. C. A view of the oroantral fistula from the oral side. D. The view of the elevated and sutured sinus mucosa. E. A view of the platelet-rich fibrin clots inserted as a membrane. F. The view of the sutured buccal advancement flap. G. A view from the oral side at two weeks postoperatively. H. A view from the oral side at two months after surgery.
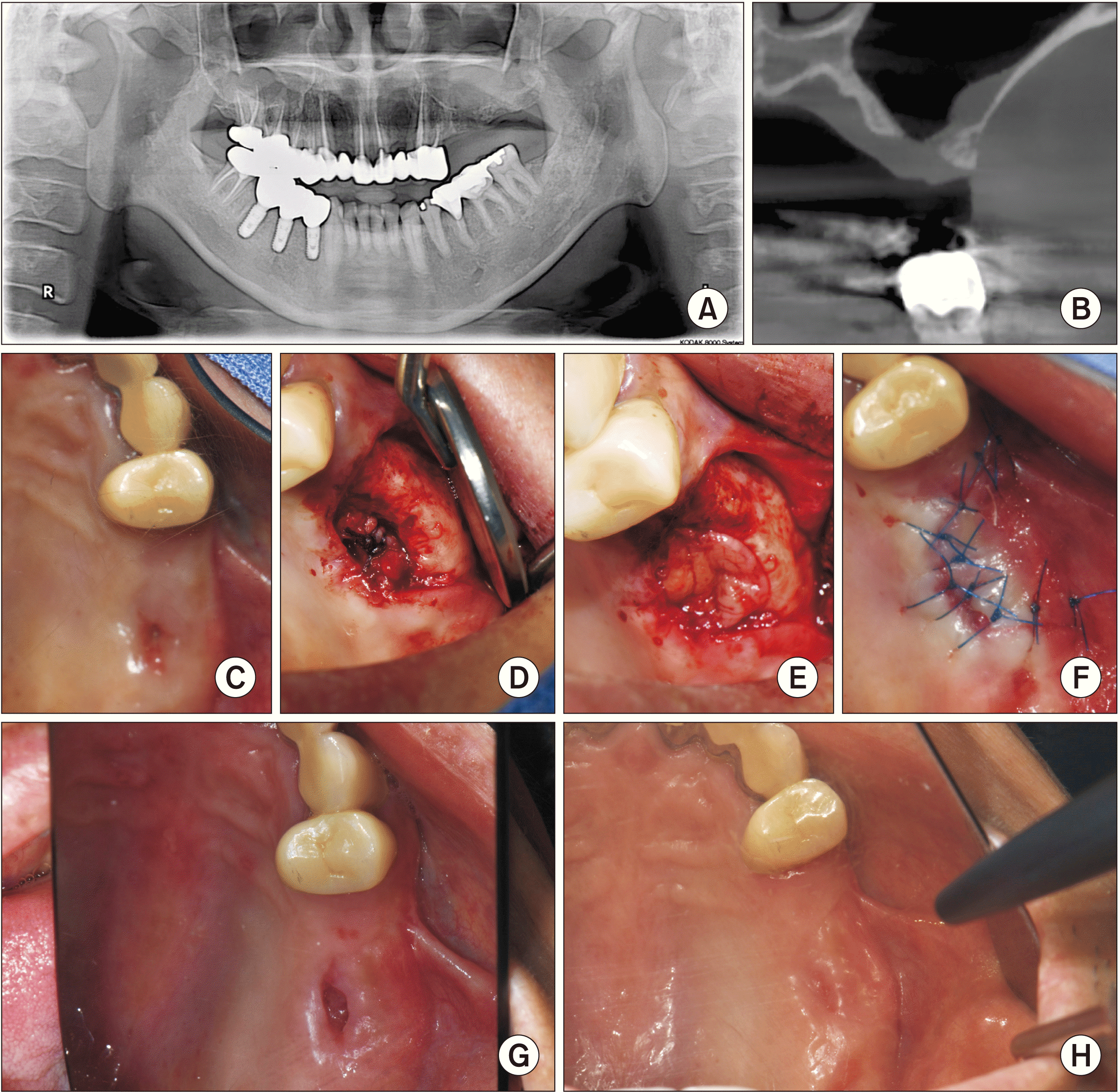
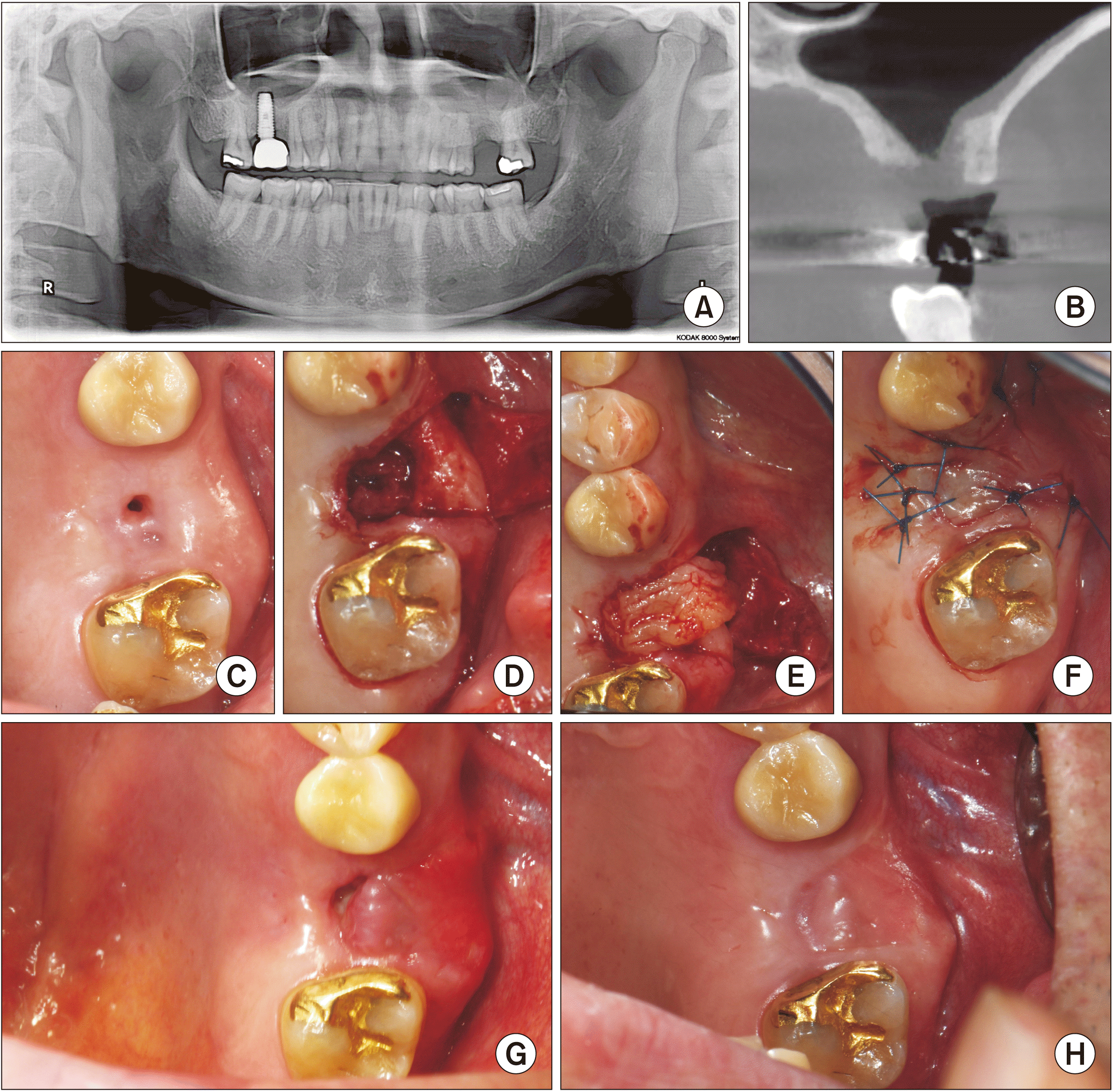
Fig. 3
A. A pre-surgery panoramic radiographs of Patient No. 2. B. The cone-beam computed tomography view. C. A view of the oroantral fistula from the oral side. D. The view of the elevated sinus mucosa. E. A view of the platelet-rich fibrin clots inserted as a membrane. F. The view of the sutured buccal advancement flap. G. A view from the oral side at two weeks postoperatively. H. A view from the oral side at two months after surgery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download